Primary Immunodeficiency Therapeutics Market Report
Published Date: 31 January 2026 | Report Code: primary-immunodeficiency-therapeutics
Primary Immunodeficiency Therapeutics Market Size, Share, Industry Trends and Forecast to 2033
This report covers the Primary Immunodeficiency Therapeutics market from 2023 to 2033, providing in-depth insights on market size, trends, technological advancements, and regional analyses to guide stakeholders in making informed decisions.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
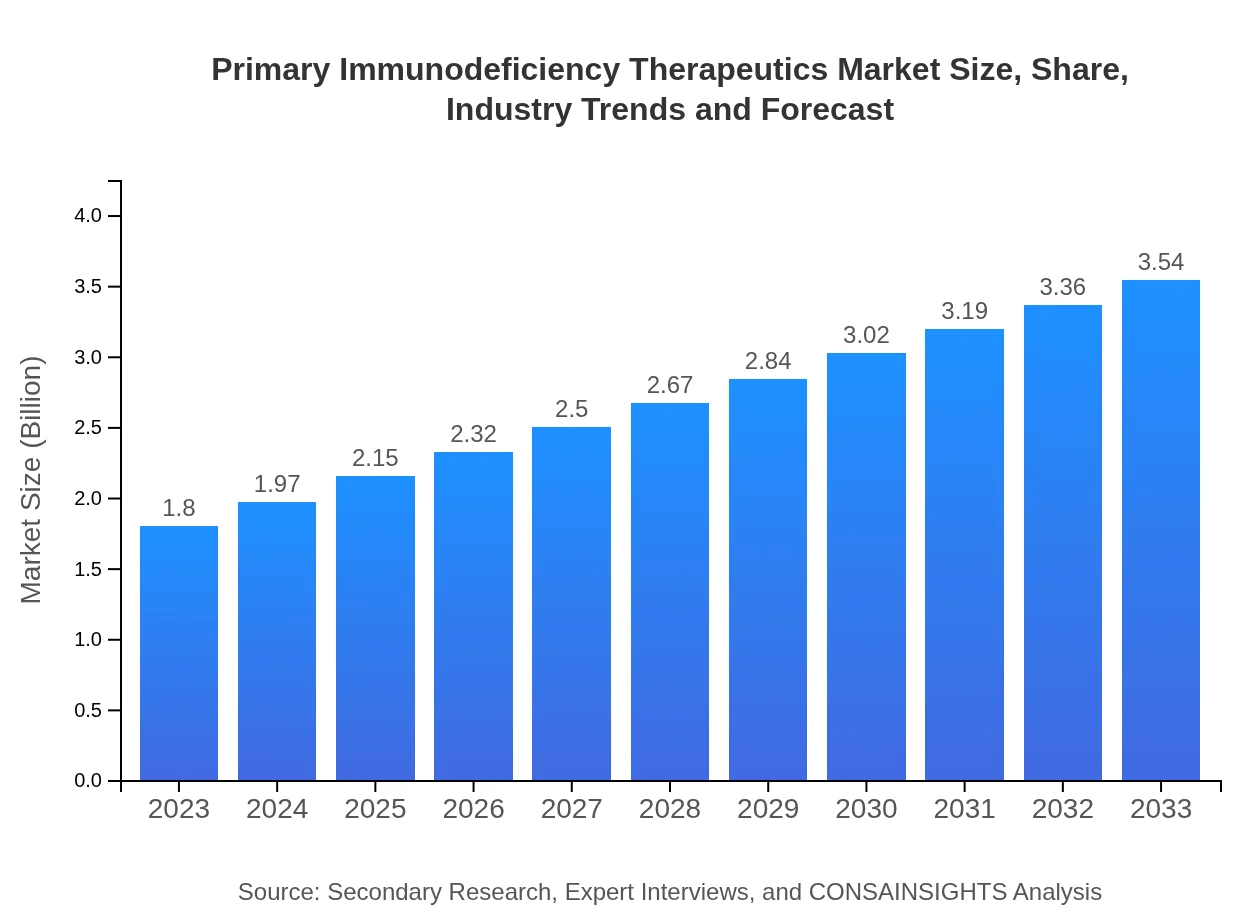
| 2023 Market Size | $1.80 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $3.54 Billion |
| Top Companies | Grifols, CSL Behring, Takeda Pharmaceutical Company, Kedrion Biopharma |
| Last Modified Date | 31 January 2026 |
Primary Immunodeficiency Therapeutics Market Overview
Customize Primary Immunodeficiency Therapeutics Market Report market research report
- ✔ Get in-depth analysis of Primary Immunodeficiency Therapeutics market size, growth, and forecasts.
- ✔ Understand Primary Immunodeficiency Therapeutics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Primary Immunodeficiency Therapeutics
What is the Market Size & CAGR of Primary Immunodeficiency Therapeutics market in 2023?
Primary Immunodeficiency Therapeutics Industry Analysis
Primary Immunodeficiency Therapeutics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Primary Immunodeficiency Therapeutics Market Analysis Report by Region
Europe Primary Immunodeficiency Therapeutics Market Report:
The European market is set to increase from USD 0.58 billion in 2023 to USD 1.14 billion by 2033, bolstered by ongoing support from various health organizations and improved genetic screening programs that facilitate early diagnosis and access to advanced therapies.Asia Pacific Primary Immunodeficiency Therapeutics Market Report:
In the Asia-Pacific region, the market is projected to grow from USD 0.33 billion in 2023 to USD 0.64 billion by 2033. Factors inspiring this growth include an increase in healthcare investments, rising awareness concerning immunodeficiency disorders, and collaborative efforts between governments and the private sector to improve healthcare access.North America Primary Immunodeficiency Therapeutics Market Report:
North America, accounting for the most substantial share, is expected to escalate from USD 0.67 billion in 2023 to USD 1.32 billion by 2033. Key contributors include advanced healthcare infrastructure, extensive research and development initiatives, and high rates of diagnosed immunodeficiency cases driving treatment demands.South America Primary Immunodeficiency Therapeutics Market Report:
In South America, the market is expected to rise modestly from USD 0.02 billion in 2023 to USD 0.05 billion by 2033. Challenges such as economic constraints and limited healthcare facilities can suppress growth, while initiatives to improve disease management and access will slowly enhance market conditions.Middle East & Africa Primary Immunodeficiency Therapeutics Market Report:
In the Middle East and Africa, the market will rise from USD 0.20 billion in 2023 to USD 0.39 billion by 2033. The growth is driven by the enhancement of healthcare settings, the need for better healthcare policies, and initiatives to raise awareness regarding immunodeficiency diseases among the population.Tell us your focus area and get a customized research report.
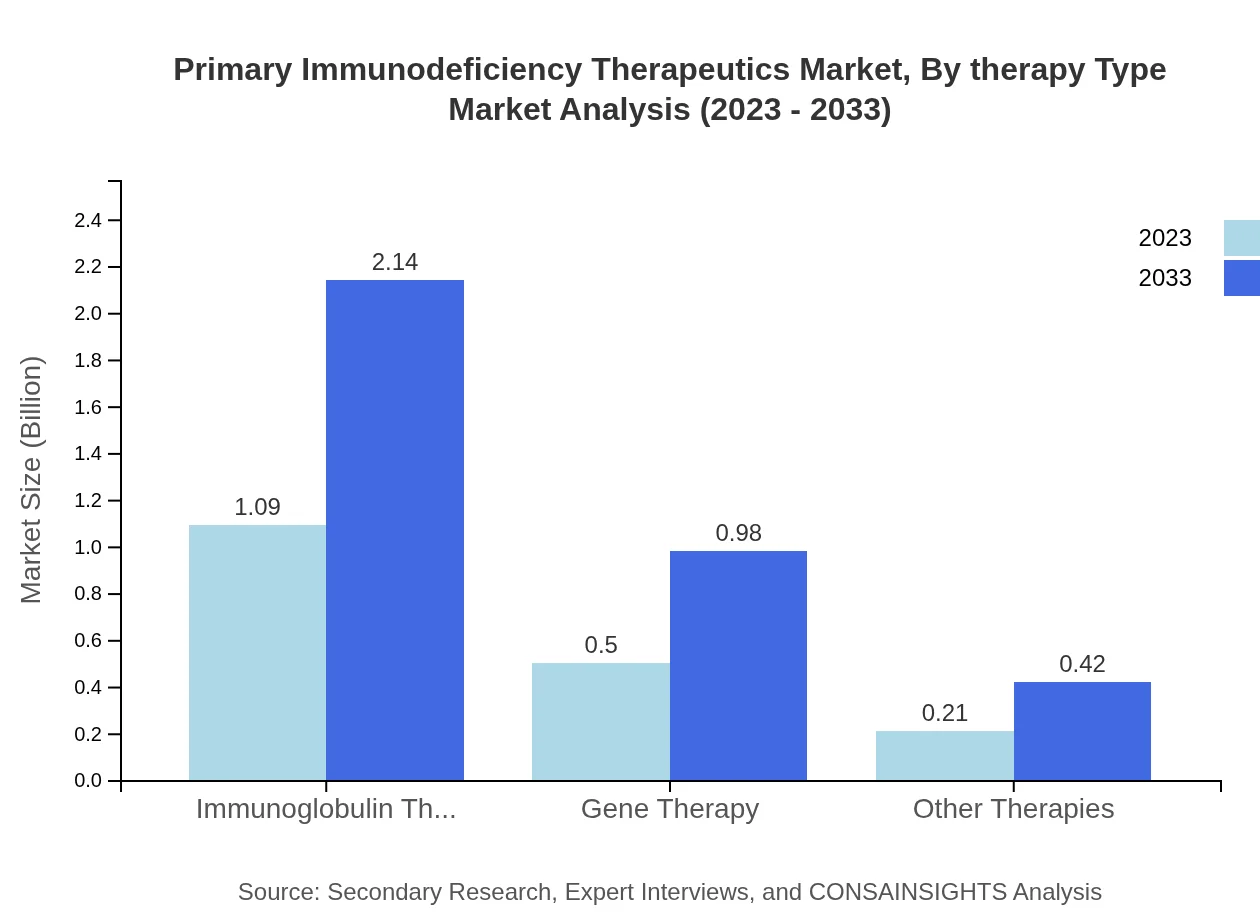
Primary Immunodeficiency Therapeutics Market Analysis By Therapy Type
The analysis of therapy types shows that Immunoglobulin Therapy, the leading segment, is projected to maintain its dominance, growing from USD 1.09 billion in 2023 to USD 2.14 billion by 2033. Gene Therapy, while smaller, holds great promise with growth from USD 0.50 billion to USD 0.98 billion by 2033. Other therapies cater to unique needs and while they represent a fraction of the market, they exhibit significant growth potential.
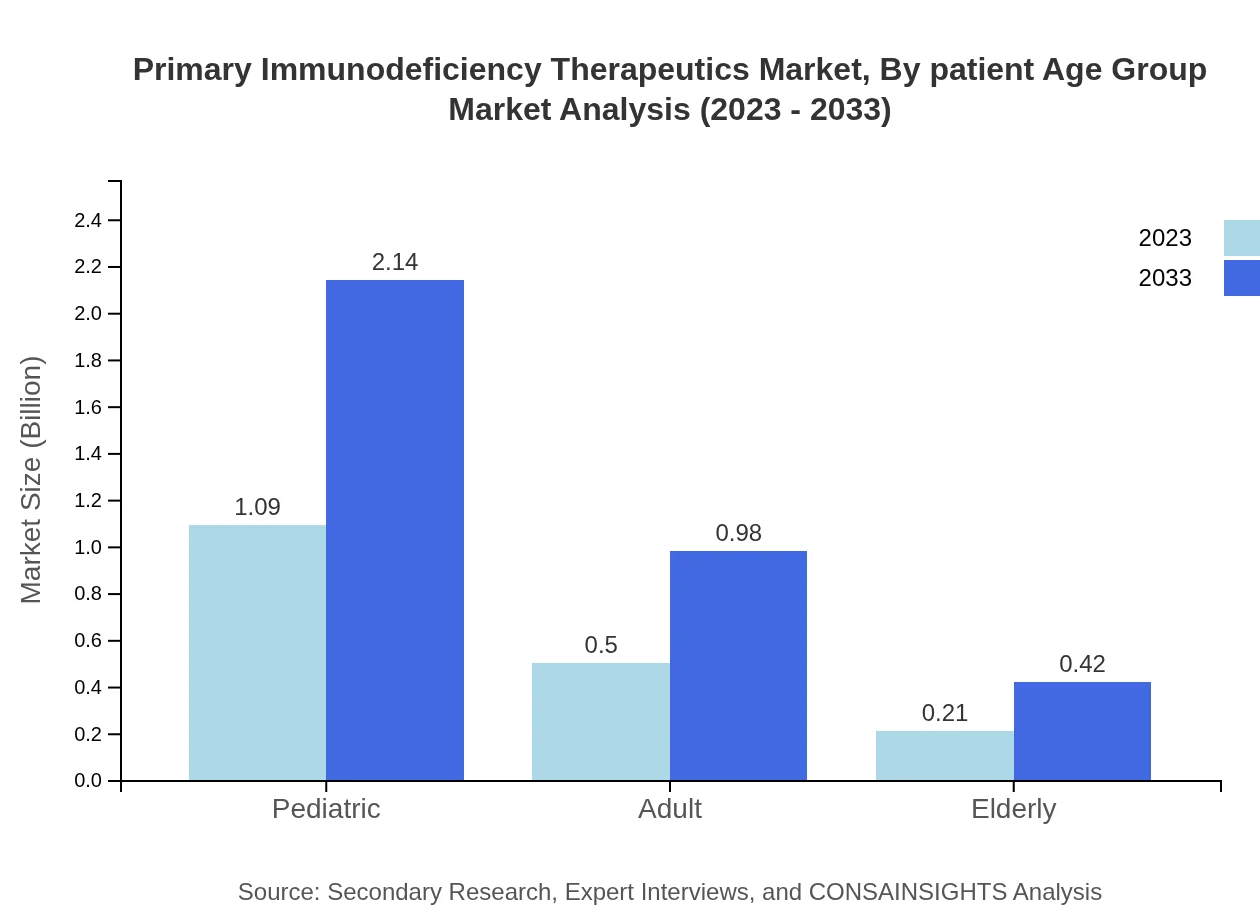
Primary Immunodeficiency Therapeutics Market Analysis By Patient Age Group
Inpatient age group segmentation shows that pediatric patients represent a significant share at 60.47% of the market. The pediatric segment is expected to expand from USD 1.09 billion to USD 2.14 billion by 2033, with adult patients also showing consistent growth. The elderly segment grows from USD 0.21 billion to USD 0.42 billion, illustrating increasing immunodeficiency concerns among older populations.
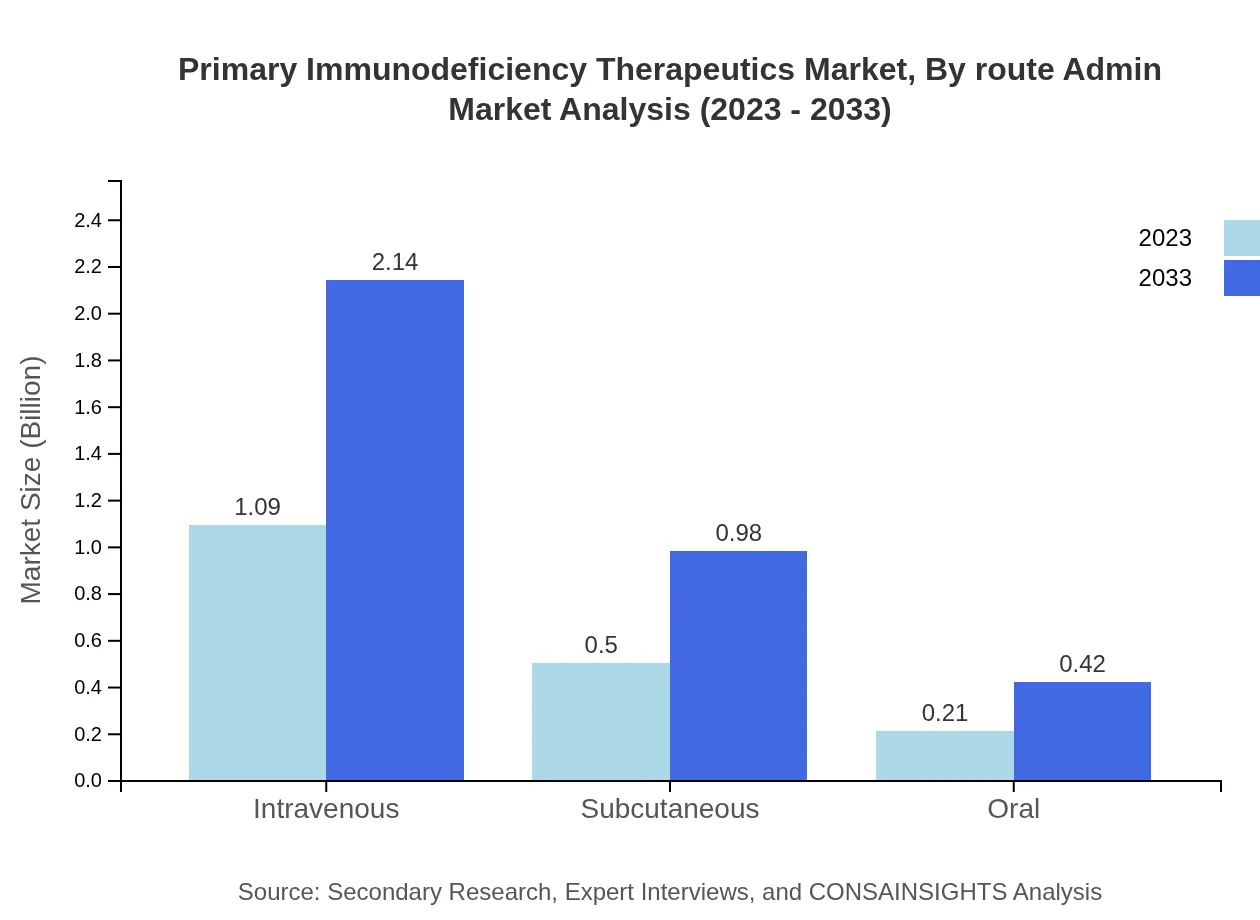
Primary Immunodeficiency Therapeutics Market Analysis By Route Admin
Byroute of administration, intravenous therapy remains the major contributor, accounting for 60.47% of market share with a growth trajectory from USD 1.09 billion to USD 2.14 billion. Subcutaneous therapies are increasingly preferred owing to their ease of administration, expected to grow from USD 0.50 billion to USD 0.98 billion. Oral therapies remain a small but growing segment, indicating a shift toward more convenient treatment options.
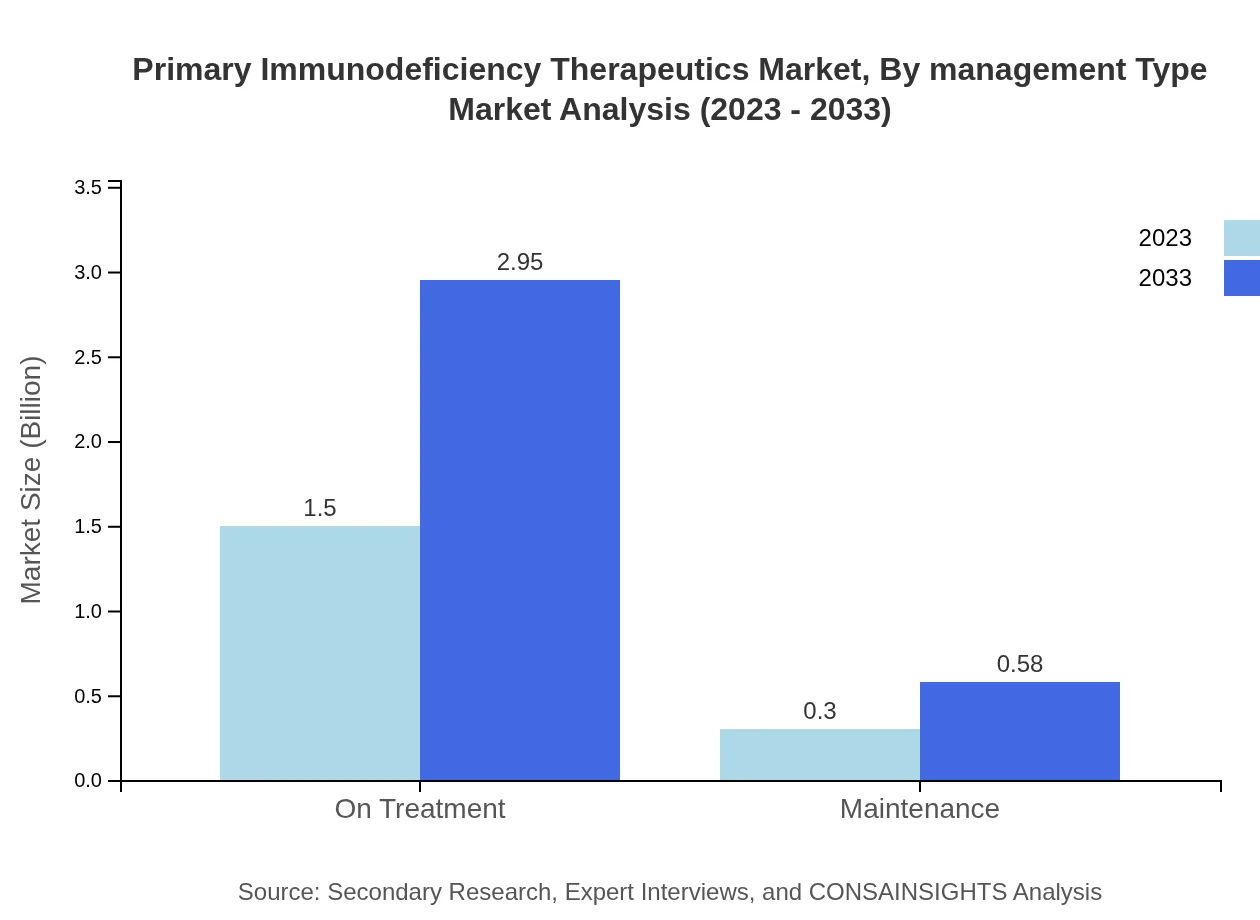
Primary Immunodeficiency Therapeutics Market Analysis By Management Type
Management types indicate that On Treatment management claims an 83.48% share of the market, extending from USD 1.50 billion in 2023 to USD 2.95 billion by 2033. Maintenance therapies serve a smaller segment, growing steadily along with advancing treatment standards, from USD 0.30 billion to USD 0.58 billion over the same period.
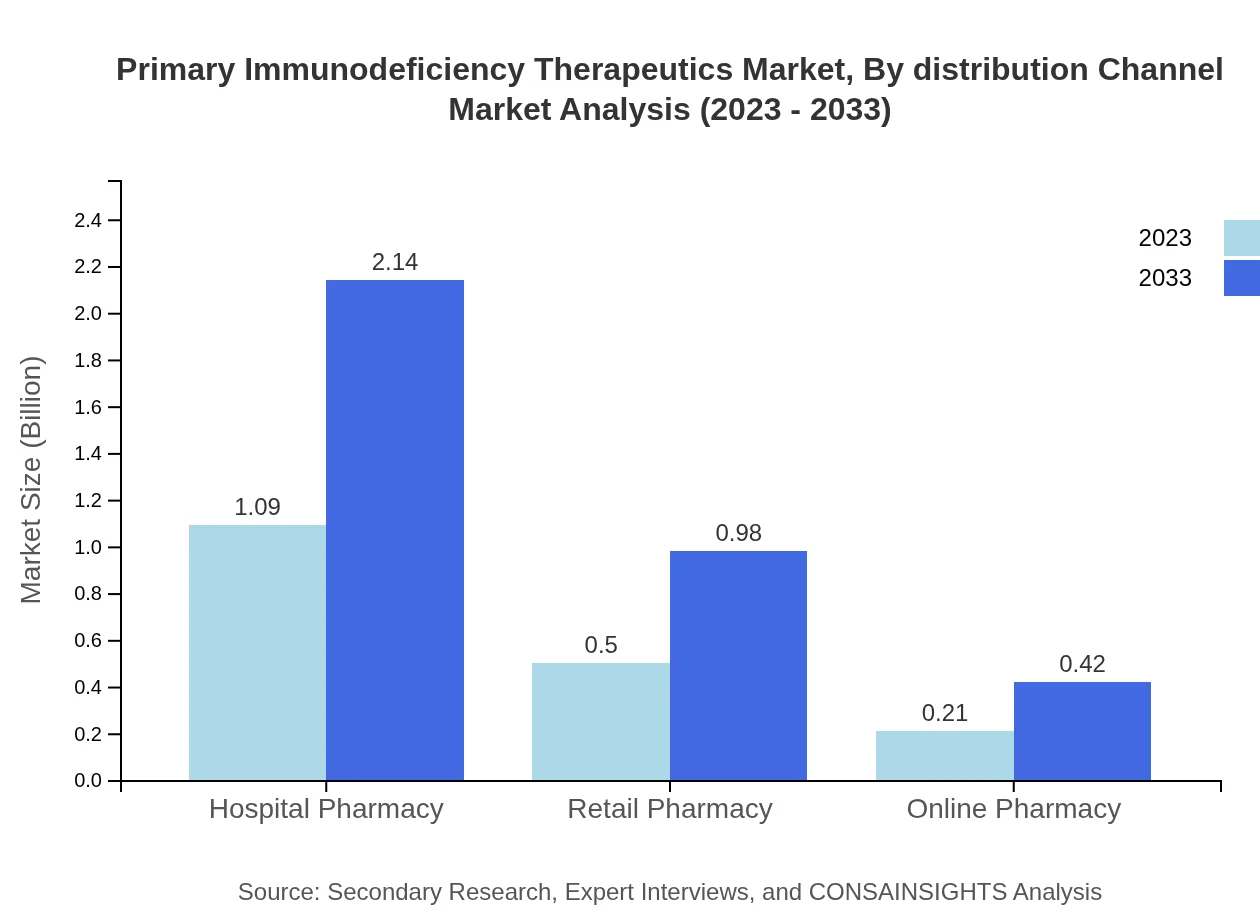
Primary Immunodeficiency Therapeutics Market Analysis By Distribution Channel
Distribution channels reveal that the hospital pharmacy sector is a dominant player, holding a share of 60.47%, which is projected to increase from USD 1.09 billion to USD 2.14 billion by 2033. Retail and online pharmacies are also significant, with shares of 27.75% and 11.78%, respectively, indicating a shift towards digital channels that complement traditional methods.
Primary Immunodeficiency Therapeutics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Primary Immunodeficiency Therapeutics Industry
Grifols:
Grifols is a global healthcare company dedicated to improving the health and well-being of people through its innovative products and services in biopharmaceuticals and diagnostics.CSL Behring:
CSL Behring specializes in biotherapies for rare and serious diseases, particularly in immunoglobulin therapies, and is a leader in managing primary immunodeficiency disorders.Takeda Pharmaceutical Company:
Takeda focuses on developing innovative therapies to enhance patient outcomes in immunology and other therapeutic areas, fostering research in immunodeficiencies.Kedrion Biopharma:
Kedrion Biopharma develops, manufactures, and markets human plasma-derived therapeutics, including immunoglobulins essential for primary immunodeficiency treatment.We're grateful to work with incredible clients.









FAQs
What is the market size of primary Immunodeficiency Therapeutics?
The primary immunodeficiency therapeutics market is valued at approximately $1.8 billion in 2023, with an anticipated compound annual growth rate (CAGR) of 6.8% projected through 2033. This growth reflects increasing awareness and advancements in treatment options.
What are the key market players or companies in this primary Immunodeficiency Therapeutics industry?
Key players in the primary immunodeficiency therapeutics market include established pharmaceutical companies and biotechnology firms that focus on innovative treatments. These market leaders continue to invest in research and development to enhance their product portfolios.
What are the primary factors driving the growth in the primary Immunodeficiency Therapeutics industry?
Factors driving market growth include increasing incidence rates of immunodeficiency disorders, advancements in treatment technologies, and rising healthcare expenditure. Additionally, patient awareness and the development of targeted therapies also significantly contribute to market expansion.
Which region is the fastest Growing in the primary Immunodeficiency Therapeutics?
North America is the fastest-growing region in the primary immunodeficiency therapeutics market. Without doubt, it is projected to grow from $0.67 billion in 2023 to $1.32 billion by 2033, reflecting robust healthcare infrastructure and increased access to innovative treatments.
Does ConsaInsights provide customized market report data for the primary Immunodeficiency Therapeutics industry?
Yes, ConsaInsights offers customized market report data tailored to specific research needs within the primary immunodeficiency therapeutics industry. This personalization helps organizations gather relevant insights crucial for strategic decision-making.
What deliverables can I expect from this primary Immunodeficiency Therapeutics market research project?
Deliverables from the primary immunodeficiency therapeutics market research project include comprehensive reports, detailed segmentation analysis, competitive landscape assessments, and actionable insights that cater to specific business objectives.
What are the market trends of primary Immunodeficiency Therapeutics?
Current trends in the primary immunodeficiency therapeutics market include the growing focus on gene therapies, innovations in immunoglobulin therapies, and a shift towards personalized medicine. These trends signify a transformation towards more effective and targeted treatment solutions.