Prophylactic Hiv Drugs Market Report
Published Date: 31 January 2026 | Report Code: prophylactic-hiv-drugs
Prophylactic Hiv Drugs Market Size, Share, Industry Trends and Forecast to 2033
This report explores the Prophylactic HIV Drugs market, providing in-depth insights on current trends, growth forecasts, and regional analyses. It spans the forecast period from 2023 to 2033, focusing on market dynamics, segmentation, and key players shaping this vital industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
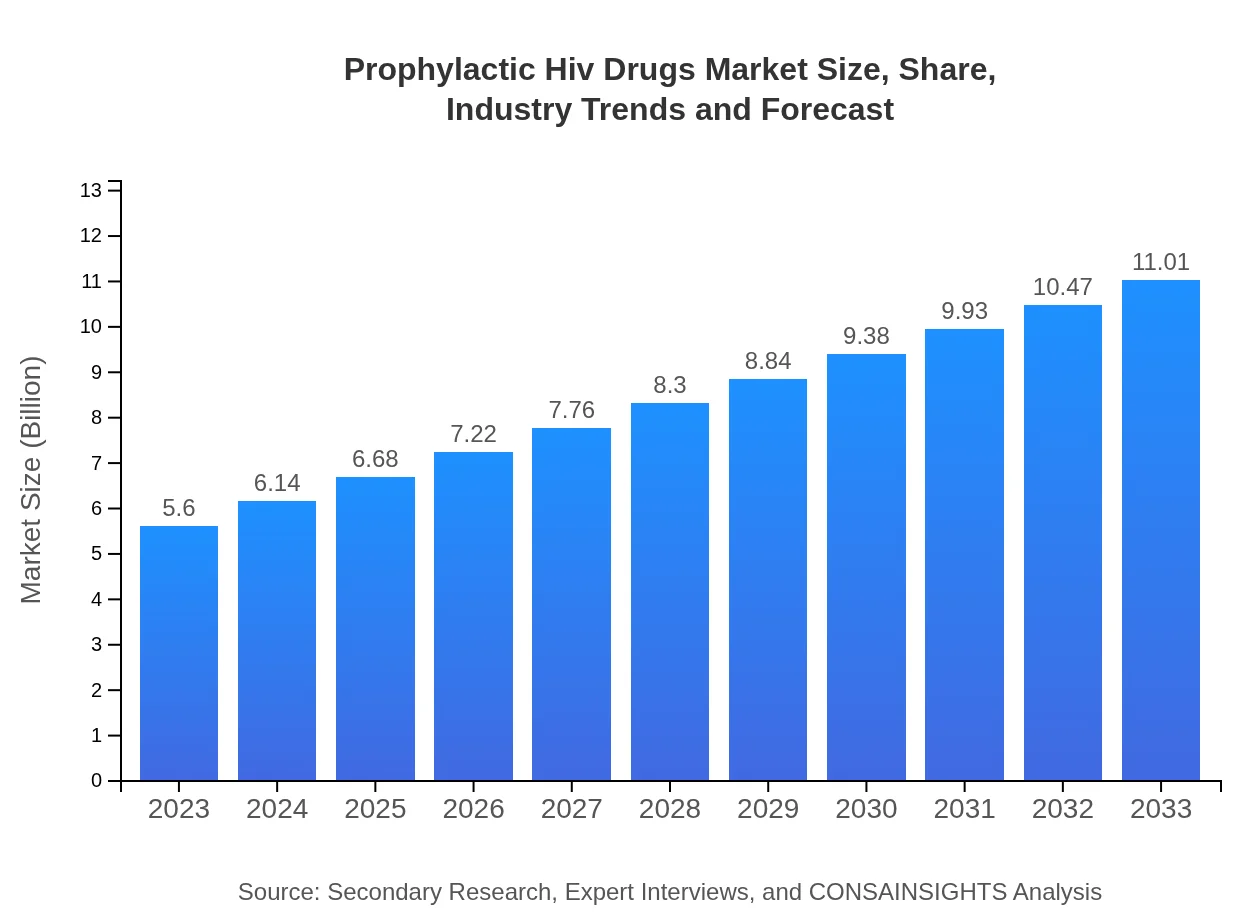
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $11.01 Billion |
| Top Companies | Gilead Sciences, ViiV Healthcare, Merck & Co., Bristol-Myers Squibb |
| Last Modified Date | 31 January 2026 |
Prophylactic Hiv Drugs Market Overview
Customize Prophylactic Hiv Drugs Market Report market research report
- ✔ Get in-depth analysis of Prophylactic Hiv Drugs market size, growth, and forecasts.
- ✔ Understand Prophylactic Hiv Drugs's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Prophylactic Hiv Drugs
What is the Market Size & CAGR of Prophylactic Hiv Drugs market in 2023?
Prophylactic Hiv Drugs Industry Analysis
Prophylactic Hiv Drugs Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Prophylactic Hiv Drugs Market Analysis Report by Region
Europe Prophylactic Hiv Drugs Market Report:
The European market is also on an upward trajectory, projected to grow from $1.53 billion in 2023 to $3.00 billion by 2033. The European region benefits from mature healthcare systems and strong support for HIV prevention initiatives.Asia Pacific Prophylactic Hiv Drugs Market Report:
The Asia Pacific region is projected to see substantial growth, with the market expected to increase from $1.09 billion in 2023 to $2.14 billion by 2033. This growth is supported by rising government initiatives and health campaigns aimed at HIV prevention, along with increasing healthcare accessibility.North America Prophylactic Hiv Drugs Market Report:
North America currently holds one of the largest markets, projected to expand from $2.05 billion in 2023 to approximately $4.03 billion by 2033. Strong healthcare infrastructure, alongside comprehensive insurance coverage for prophylactic treatments, contributes to this growth.South America Prophylactic Hiv Drugs Market Report:
In South America, the market size is expected to grow from $0.44 billion in 2023 to $0.87 billion by 2033. The increasing prevalence of HIV, combined with enhanced accessibility to prophylactic treatments, is expected to drive this growth.Middle East & Africa Prophylactic Hiv Drugs Market Report:
The Middle East and Africa market, though smaller, shows potential growth from $0.49 billion in 2023 to $0.97 billion by 2033, driven by increased healthcare spending and focused campaigns on AIDS awareness and prevention.Tell us your focus area and get a customized research report.
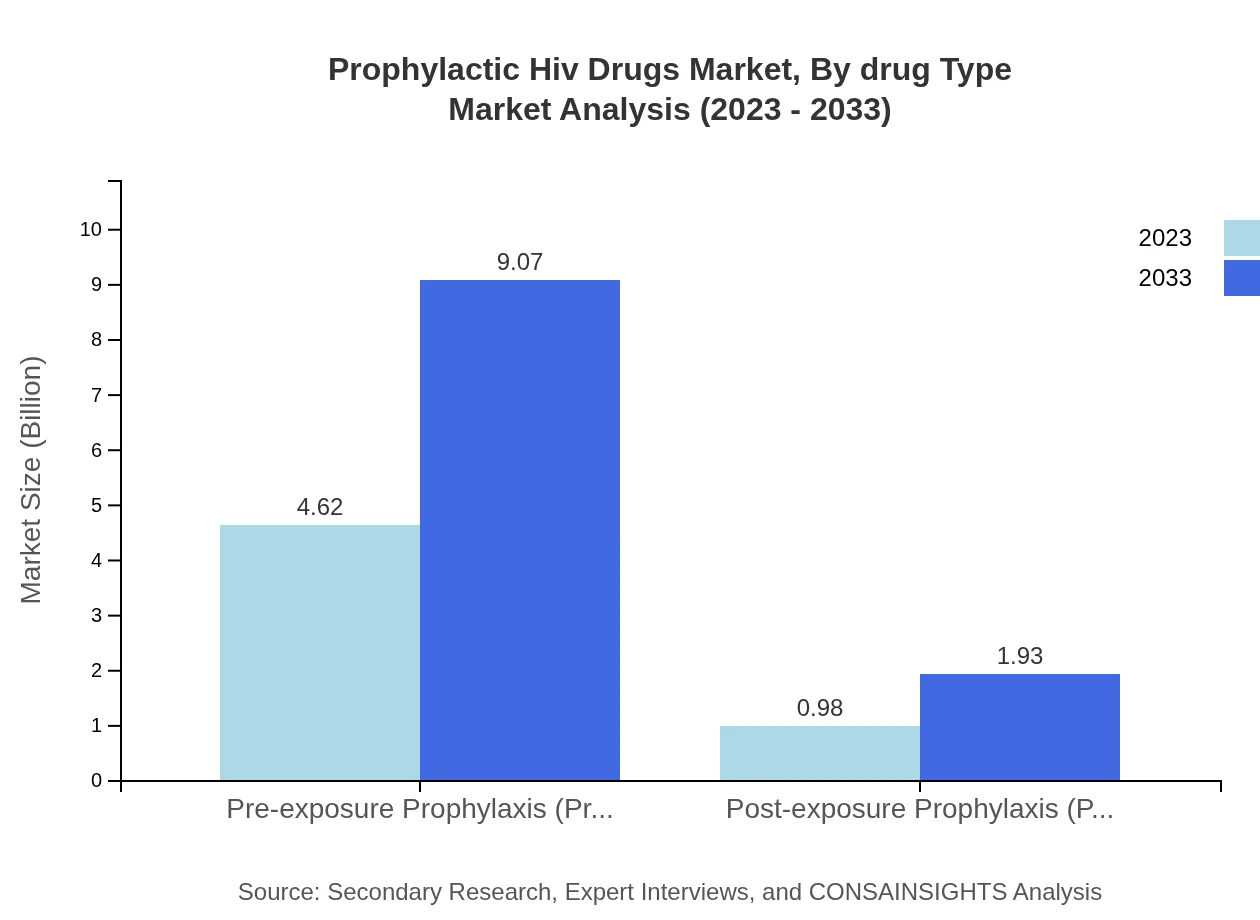
Prophylactic Hiv Drugs Market Analysis By Drug Type
PrEP dominates the market, accounting for 82.45% of the share in 2023, expected to maintain this lead through 2033. PEP, while smaller, comprises 17.55% of the market, indicating its role in post-exposure scenarios. Continued education around these options will enhance usage rates.
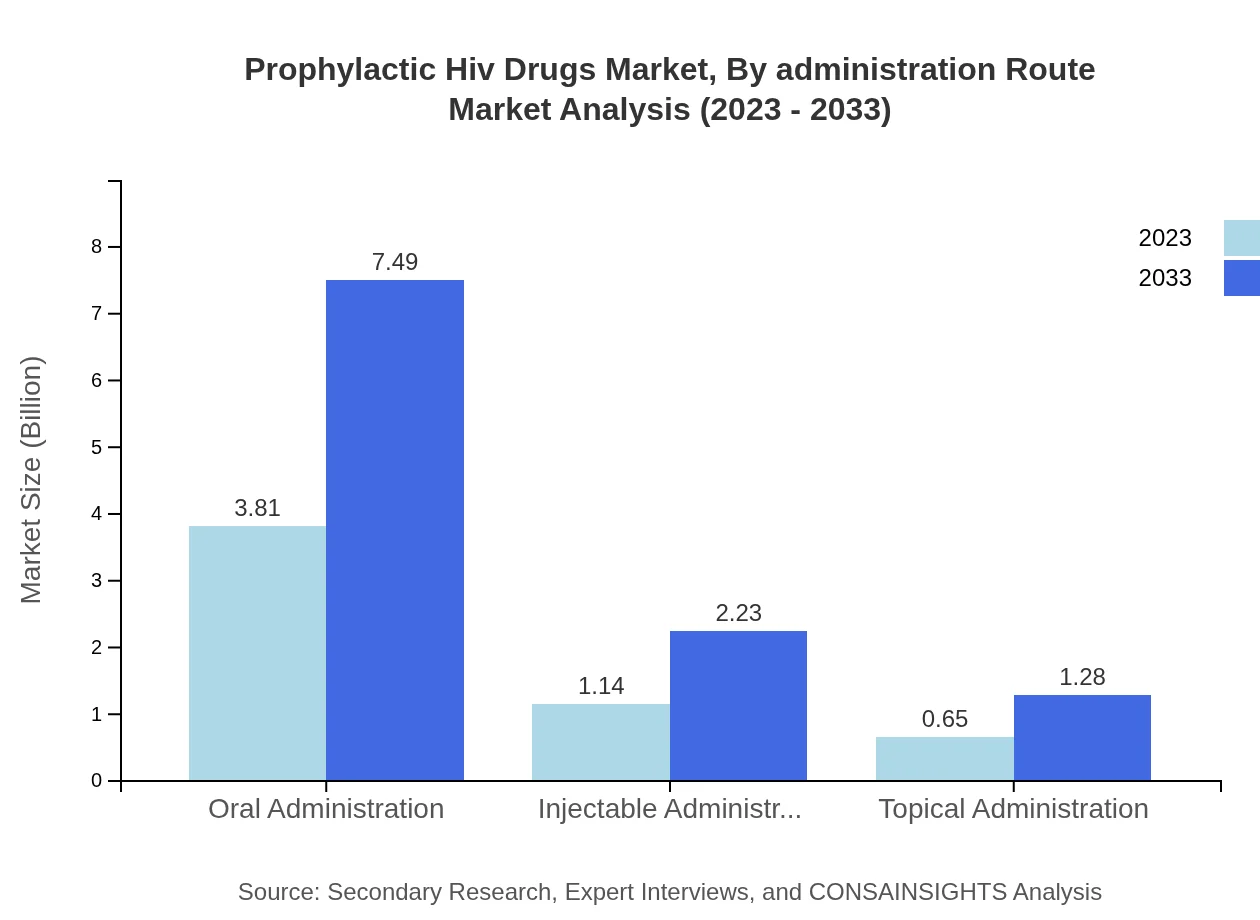
Prophylactic Hiv Drugs Market Analysis By Administration Route
Oral administration accounts for the largest share of 68.07% in 2023, with growth projected alongside improved formulations. Injectable and topical routes, making up 20.29% and 11.64% respectively, provide alternative options that cater to diverse user preferences.
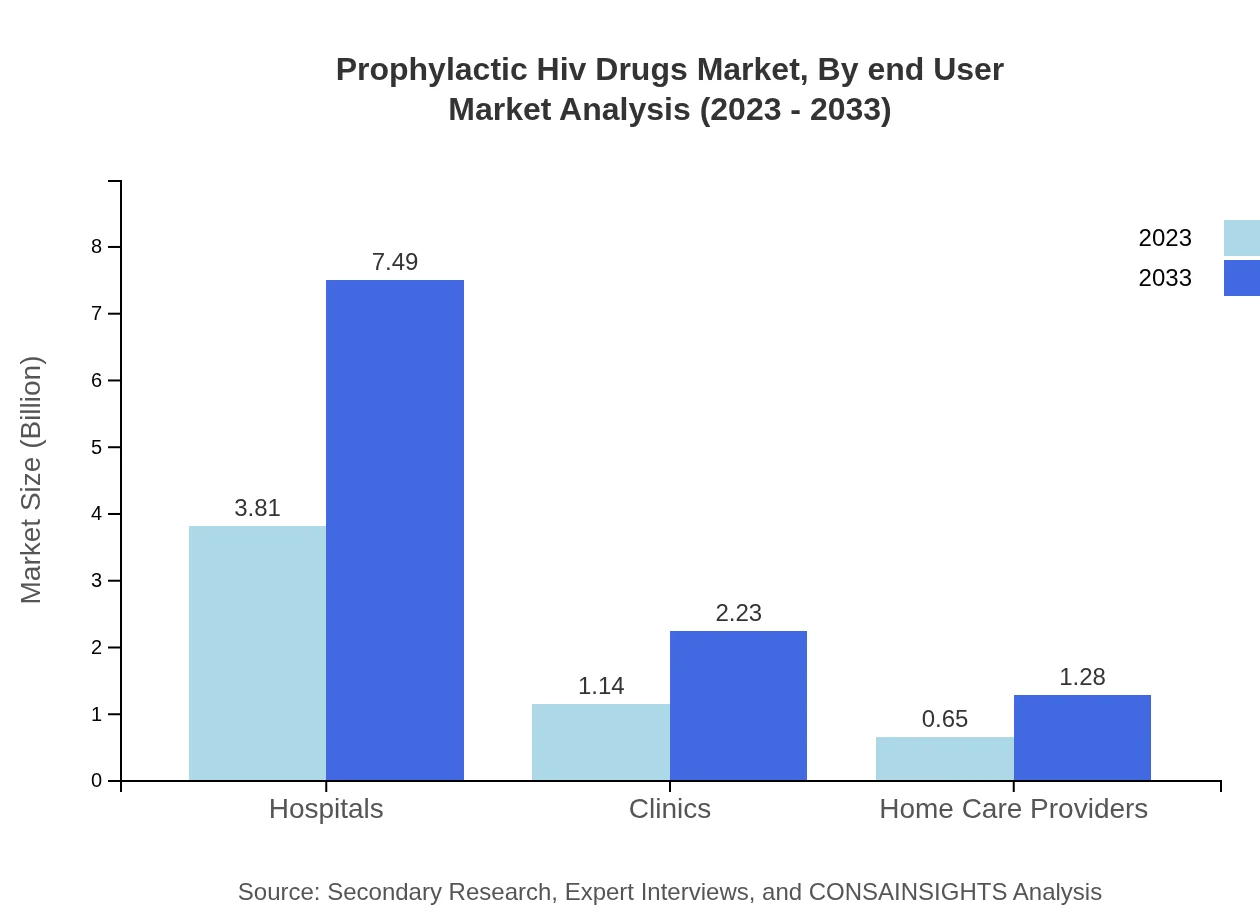
Prophylactic Hiv Drugs Market Analysis By End User
Hospitals represent the largest segment, with a share of 68.07% in 2023. Clinics, making up 20.29%, and home care providers at 11.64%, play essential roles in expanding access to prophylactic drugs.
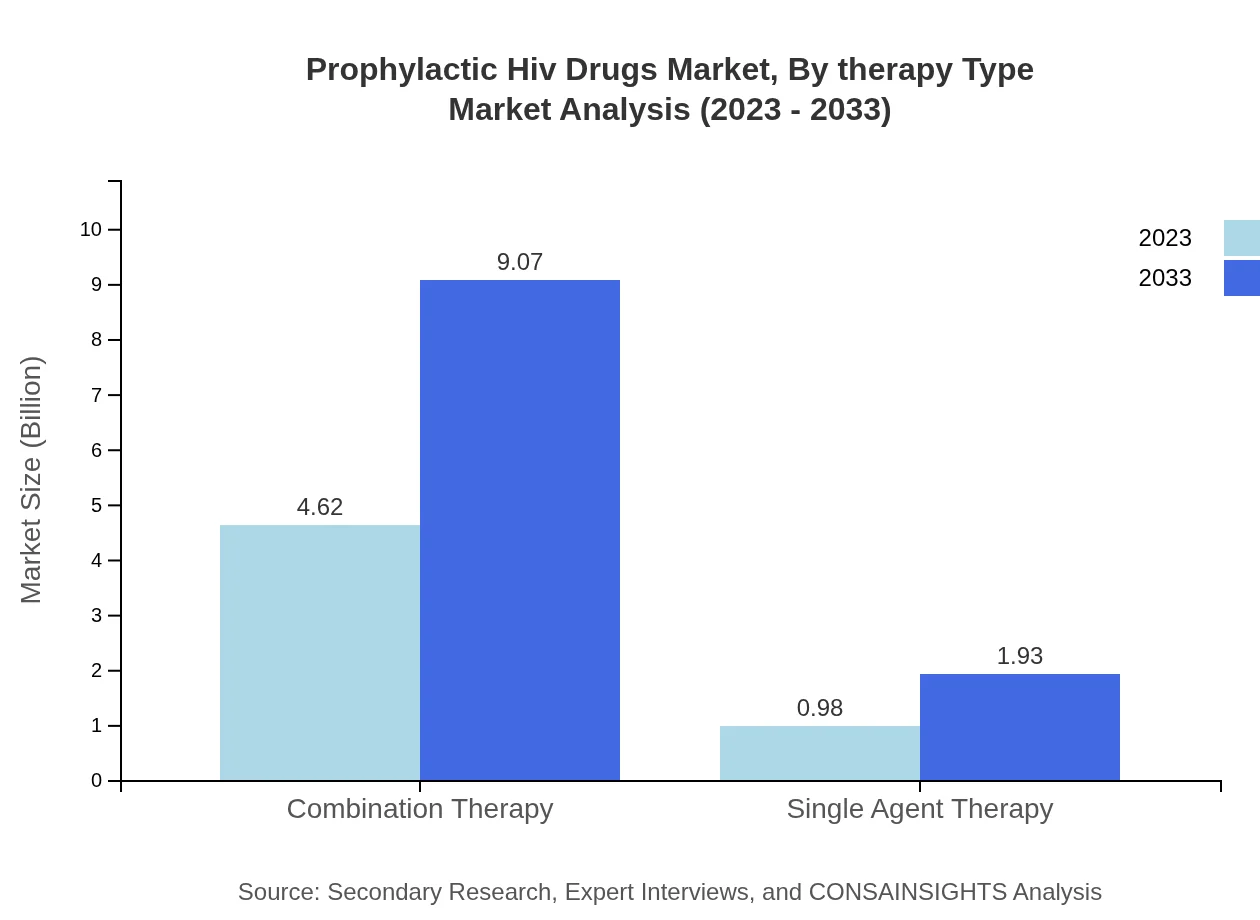
Prophylactic Hiv Drugs Market Analysis By Therapy Type
Combination therapy leads the market with a significant share of 82.45% in 2023, as healthcare providers increasingly favor multi-drug regimens. Single agent therapy, accounting for 17.55%, remains relevant in specific clinical contexts.
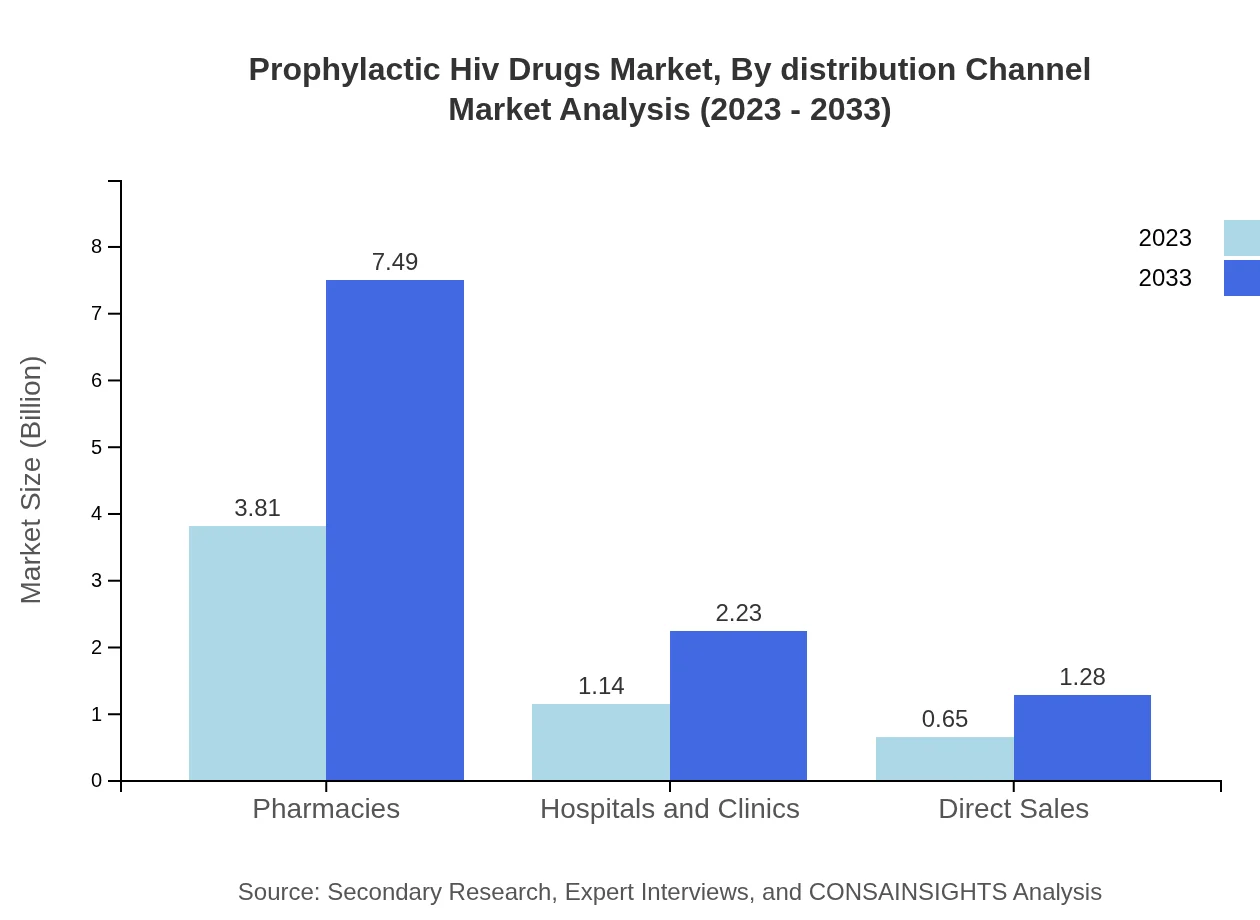
Prophylactic Hiv Drugs Market Analysis By Distribution Channel
Pharmacies play a pivotal role, controlling 68.07% of the distribution market in 2023. Hospitals and clinics contribute 20.29%, while direct sales account for 11.64%, highlighting the importance of varied distribution methods in maintaining drug accessibility.
Prophylactic Hiv Drugs Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Prophylactic Hiv Drugs Industry
Gilead Sciences:
A leader in antiviral drug development, Gilead is renowned for its innovations in PrEP, particularly with the drug Truvada, paving the way for effective HIV prevention.ViiV Healthcare:
Focused on HIV treatments, ViiV plays a crucial role in developing both PEP and PrEP options, contributing significantly to global access to preventative therapies.Merck & Co.:
Merck is known for its diverse portfolio of medicines, including contributions to HIV prevention and treatment through innovative therapies and public health partnerships.Bristol-Myers Squibb:
This pharmaceutical company is involved in HIV research and development, focusing on both therapeutic and preventive strategies to combat HIV transmission.We're grateful to work with incredible clients.









FAQs
What is the market size of prophylactic Hiv Drugs?
The prophylactic HIV drugs market size is projected to be $5.6 billion in 2023, with a strong CAGR of 6.8% leading up to 2033. This growth highlights increasing awareness and demand for preventive measures against HIV.
What are the key market players or companies in this prophylactic Hiv Drugs industry?
Key market players in the prophylactic HIV drugs industry include major pharmaceutical companies recognized for their innovative drug development, ranging from established giants to emerging biotech firms focused on HIV prevention solutions.
What are the primary factors driving the growth in the prophylactic Hiv Drugs industry?
Growth in the prophylactic HIV drugs industry is driven by rising awareness of HIV preventive measures, increasing prevalence of HIV, advancements in medication effectiveness, and supportive governmental policies encouraging drug accessibility.
Which region is the fastest Growing in the prophylactic Hiv Drugs?
The fastest-growing region for prophylactic HIV drugs is North America, with a market size projected to grow from $2.05 billion in 2023 to $4.03 billion by 2033. Other notable regions include Europe and Asia Pacific.
Does ConsaInsights provide customized market report data for the prophylactic Hiv Drugs industry?
Yes, ConsaInsights offers customized market report data specifically tailored to clients’ needs in the prophylactic HIV drugs industry, including in-depth insights, forecasts, and analysis to support strategic decision-making.
What deliverables can I expect from this prophylactic Hiv Drugs market research project?
From this market research project, clients can expect comprehensive deliverables including detailed market analysis, growth forecasts, segmentation data, competitive landscape insights, and strategic recommendations applicable to the prophylactic HIV drugs market.
What are the market trends of prophylactic Hiv Drugs?
Current market trends in prophylactic HIV drugs include increased adoption of pre-exposure prophylaxis (PrEP), innovative drug formulations, public health initiatives promoting preventive care, and enhanced distribution channels across regions.