Protein Stability Analysis Market Report
Published Date: 31 January 2026 | Report Code: protein-stability-analysis
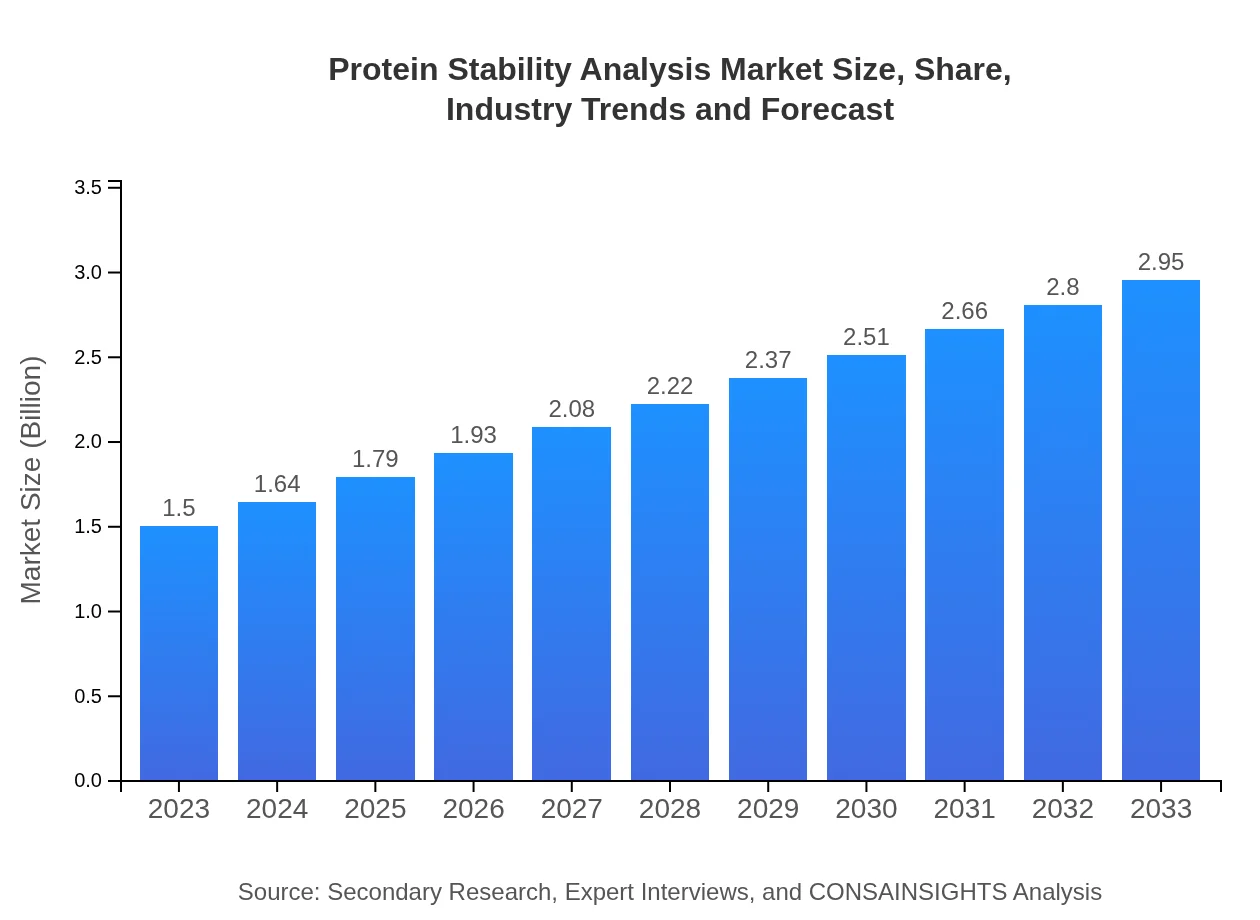
Protein Stability Analysis Market Size, Share, Industry Trends and Forecast to 2033
This report provides an extensive insight into the Protein Stability Analysis market, featuring key market trends, competitive landscape, and projections for 2023 to 2033. It details market size, segmentation, regional analysis, and technological advancements affecting the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $1.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $2.95 Billion |
| Top Companies | Thermo Fisher Scientific, Waters Corporation, Agilent Technologies, PerkinElmer, Merck KGaA |
| Last Modified Date | 31 January 2026 |
Protein Stability Analysis Market Overview
Customize Protein Stability Analysis Market Report market research report
- ✔ Get in-depth analysis of Protein Stability Analysis market size, growth, and forecasts.
- ✔ Understand Protein Stability Analysis's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Protein Stability Analysis
What is the Market Size & CAGR of Protein Stability Analysis market in 2023 and 2033?
Protein Stability Analysis Industry Analysis
Protein Stability Analysis Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Protein Stability Analysis Market Analysis Report by Region
Europe Protein Stability Analysis Market Report:
The European market for Protein Stability Analysis is expected to rise from $0.44 billion in 2023 to $0.86 billion by 2033. Stringent regulations and an emphasis on quality and safety standards in pharmaceuticals are key growth factors.Asia Pacific Protein Stability Analysis Market Report:
The Asia Pacific region is witnessing a surge in the Protein Stability Analysis market, projected to grow from $0.29 billion in 2023 to $0.56 billion by 2033. An increase in pharmaceutical R&D initiatives, coupled with growing investments in biotechnology, is a significant driver.North America Protein Stability Analysis Market Report:
North America remains a dominant player in the Protein Stability Analysis market, projected to grow from $0.55 billion in 2023 to $1.08 billion by 2033. The region's advanced healthcare infrastructure, robust investment in drug development, and strong presence of pharmaceutical companies drive this growth.South America Protein Stability Analysis Market Report:
In South America, the market is expected to expand from $0.15 billion in 2023 to $0.29 billion by 2033. Increasing healthcare spending and the growing focus on biotechnology are influencing market dynamics positively.Middle East & Africa Protein Stability Analysis Market Report:
The Middle East and Africa market is forecasted to grow from $0.08 billion in 2023 to $0.16 billion by 2033. Growth in this region is supported by increased healthcare initiatives and adoption of biopharmaceuticals.Tell us your focus area and get a customized research report.
Protein Stability Analysis Market Analysis Pharmaceutical_companies
Global Protein Stability Analysis Market, By Pharmaceutical Companies (2023 - 2033)
In 2023, the market for pharmaceutical companies in Protein Stability Analysis is valued at $0.91 billion and will rise to $1.78 billion by 2033. This segment holds a significant share of 60.36%, reflecting the essential nature of stability analysis in drug formulation.
Protein Stability Analysis Market Analysis Research_institutes
Global Protein Stability Analysis Market, By Research Institutes (2023 - 2033)
Research institutes are expected to witness growth from $0.37 billion in 2023 to $0.72 billion by 2033, holding approximately 24.49% of the market share, as they increasingly adopt stability analysis for academic and clinical research.
Protein Stability Analysis Market Analysis Contract_research_organizations
Global Protein Stability Analysis Market, By Contract Research Organizations (2023 - 2033)
The contract research organizations segment is projected to grow from $0.23 billion in 2023 to $0.45 billion in 2033, contributing around 15.15% to the market share. This growth is facilitated by increased outsourcing in pharmaceutical development.
Protein Stability Analysis Market Analysis Drug_development
Global Protein Stability Analysis Market, By Drug Development (2023 - 2033)
For drug development, the market size is expected to grow from $0.83 billion in 2023 to $1.63 billion by 2033, reflecting a share of 55.13%. The demand for stability analysis during the phases of drug development is on the rise.
Protein Stability Analysis Market Analysis Biotechnology
Global Protein Stability Analysis Market, By Biotechnology (2023 - 2033)
Biotechnology applications are expected to rise from $0.35 billion in 2023 to $0.68 billion by 2033, making up about 23.14% of the market. The growing biotech sector is amplifying the need for accurate stability testing.
Protein Stability Analysis Market Analysis Academic_research
Global Protein Stability Analysis Market, By Academic Research (2023 - 2033)
The academic research sector will grow from $0.16 billion in 2023 to $0.31 billion by 2033, representing a 10.43% market share, as educational institutions leverage stability analysis in their research programs.
Protein Stability Analysis Market Analysis Diagnostics
Global Protein Stability Analysis Market, By Diagnostics (2023 - 2033)
The diagnostics segment is expected to increase from $0.17 billion in 2023 to $0.33 billion by 2033, holding 11.3% of the market, driven by the growing need for reliable diagnostic tools.
Protein Stability Analysis Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Protein Stability Analysis Industry
Thermo Fisher Scientific:
A leading provider of scientific instrumentation, reagents, and consumables that contributes significantly to protein stability analysis tools.Waters Corporation:
Waters is known for its innovative measurement technologies and supports the Protein Stability Analysis industry with advanced analytical solutions.Agilent Technologies:
Agilent offers a broad portfolio of laboratory instruments and services utilized in the assessment of protein stability.PerkinElmer:
Providing a range of analytical instrumentation and services, PerkinElmer plays a pivotal role in protein stability analysis.Merck KGaA:
Merck offers a variety of solutions tailored for stability analysis in protein therapeutics, bolstering the industry with quality standards.We're grateful to work with incredible clients.









FAQs
What is the market size of protein Stability Analysis?
The protein stability analysis market is valued at approximately $1.5 billion in 2023, with an expected compound annual growth rate (CAGR) of 6.8% over the decade, projecting significant growth by 2033.
What are the key market players or companies in this protein Stability Analysis industry?
Key players in the protein stability analysis market include leading pharmaceutical companies, research institutes, and contract research organizations that drive innovations and developments in this field.
What are the primary factors driving the growth in the protein Stability Analysis industry?
Growth in the protein stability analysis industry is driven by increasing demand for drug development and biopharmaceutical research, along with advancements in analytical technologies and rising investments in R&D.
Which region is the fastest Growing in the protein Stability Analysis?
North America is the fastest-growing region for protein stability analysis, expected to rise from $0.55 billion in 2023 to $1.08 billion in 2033, followed closely by Europe and Asia Pacific.
Does ConsaInsights provide customized market report data for the protein Stability Analysis industry?
Yes, Consainsights offers customized market reports tailored to specific needs within the protein stability analysis industry for in-depth insights and strategic planning.
What deliverables can I expect from this protein Stability Analysis market research project?
Deliverables from our research project include detailed reports on market size, trends, segmentation analyses, regional insights, and competitive landscapes to guide your strategy.
What are the market trends of protein Stability Analysis?
Current trends in the protein stability analysis market include an increasing focus on biopharmaceuticals, usage of advanced experimental and computational methods, and rising collaborations among key industry players.