Psoriasis Therapeutics Market Report
Published Date: 31 January 2026 | Report Code: psoriasis-therapeutics
Psoriasis Therapeutics Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Psoriasis Therapeutics market, covering industry trends, market size, segmentation, and regional insights from 2023 to 2033. It offers valuable data that can aid in strategic planning and forecasting.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
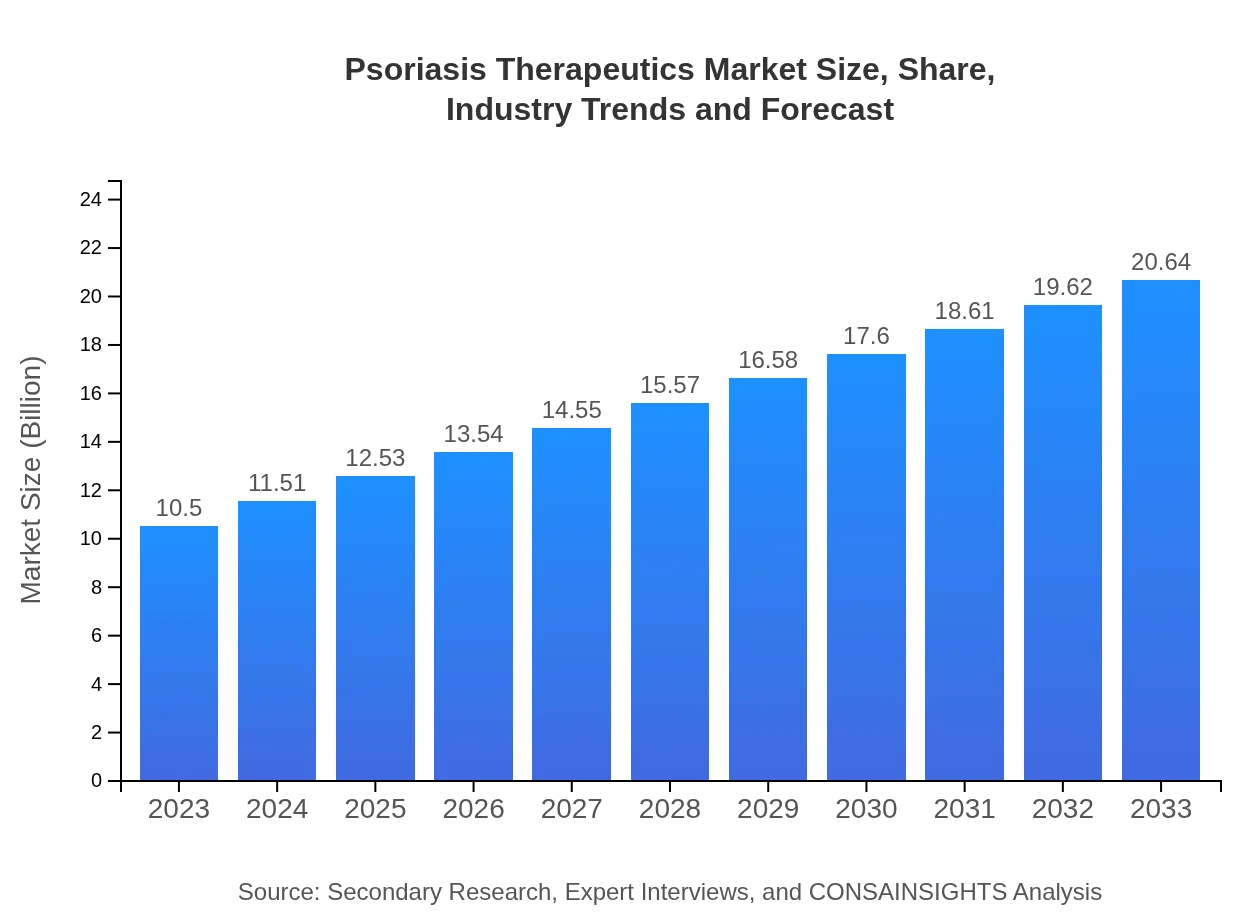
| 2023 Market Size | $10.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $20.64 Billion |
| Top Companies | AbbVie Inc., Amgen Inc., Johnson & Johnson., Novartis AG. |
| Last Modified Date | 31 January 2026 |
Psoriasis Therapeutics Market Overview
Customize Psoriasis Therapeutics Market Report market research report
- ✔ Get in-depth analysis of Psoriasis Therapeutics market size, growth, and forecasts.
- ✔ Understand Psoriasis Therapeutics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Psoriasis Therapeutics
What is the Market Size & CAGR of Psoriasis Therapeutics market in 2023 and 2033?
Psoriasis Therapeutics Industry Analysis
Psoriasis Therapeutics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Psoriasis Therapeutics Market Analysis Report by Region
Europe Psoriasis Therapeutics Market Report:
In Europe, the market was approximately $2.64 billion in 2023 and is projected to reach $5.20 billion by 2033. The European market's growth is driven by increasing healthcare investments and a high prevalence of skin disorders.Asia Pacific Psoriasis Therapeutics Market Report:
In 2023, the Psoriasis Therapeutics market in the Asia Pacific region was valued at $2.03 billion, anticipated to reach $4.00 billion by 2033. The increase is driven by rising prevalence, improved healthcare access, and the integration of new treatment guidelines in emerging economies.North America Psoriasis Therapeutics Market Report:
North America dominates the market with a value of $3.83 billion in 2023, expected to expand to $7.53 billion by 2033. Greater insurance coverage for psoriasis treatments and the rapidly advancing therapeutic landscape fuel this growth.South America Psoriasis Therapeutics Market Report:
The market in South America was valued at $0.90 billion in 2023 and is projected to grow to $1.77 billion by 2033. This growth is aided by the increasing awareness of psoriasis and the availability of new treatment options in urban areas.Middle East & Africa Psoriasis Therapeutics Market Report:
The market in the Middle East and Africa was valued at $1.09 billion in 2023, anticipated to grow to $2.14 billion by 2033. Increasing awareness and improved healthcare infrastructure are significant contributors to this growth.Tell us your focus area and get a customized research report.
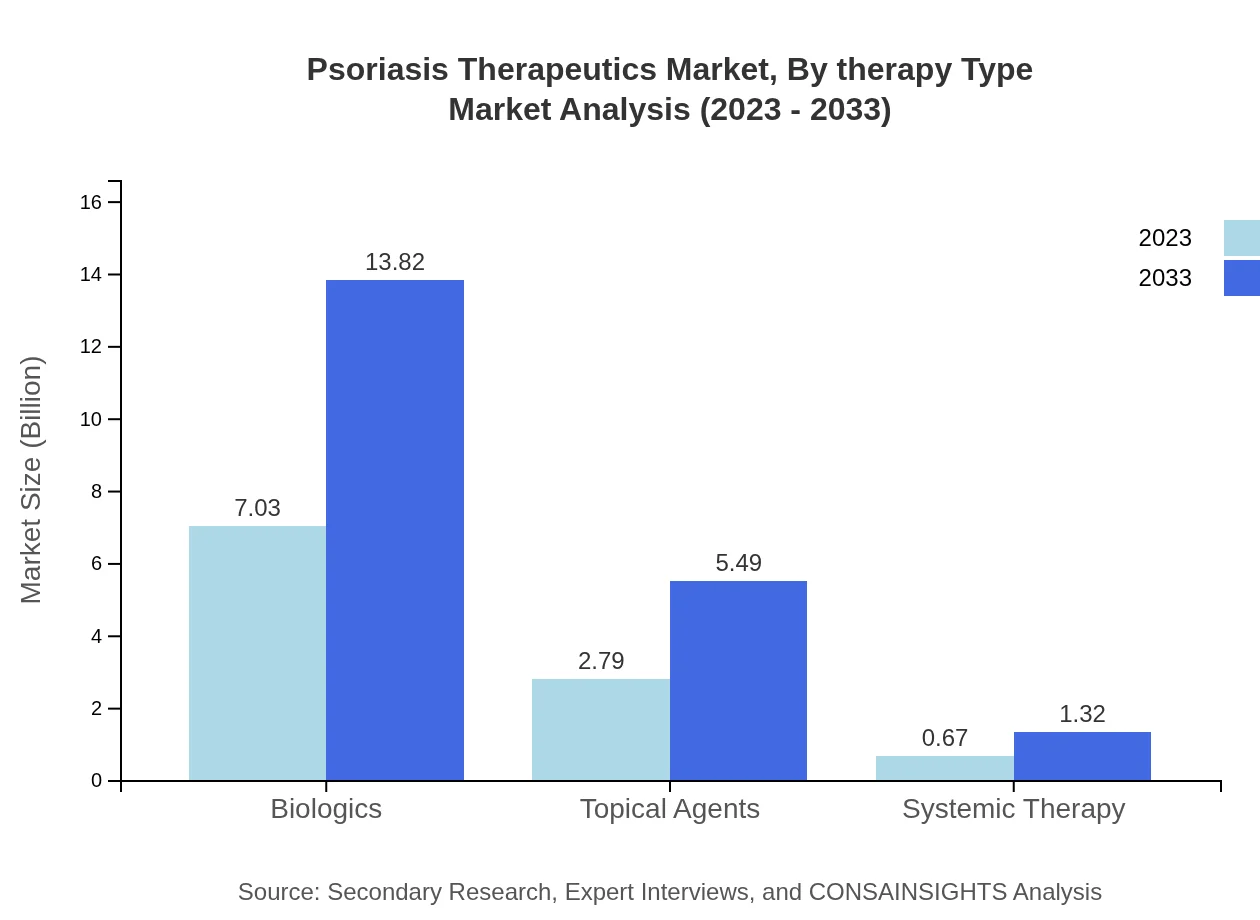
Psoriasis Therapeutics Market Analysis By Therapy Type
In 2023, the Biologics segment holds a market share of 66.99% with a value of $7.03 billion, projected to reach $13.82 billion by 2033. Topical agents follow with a market share of 26.59%, and Systemic therapy accounts for 6.42%. These segments highlight the preference for biologic treatments due to their targeted action and efficacy.
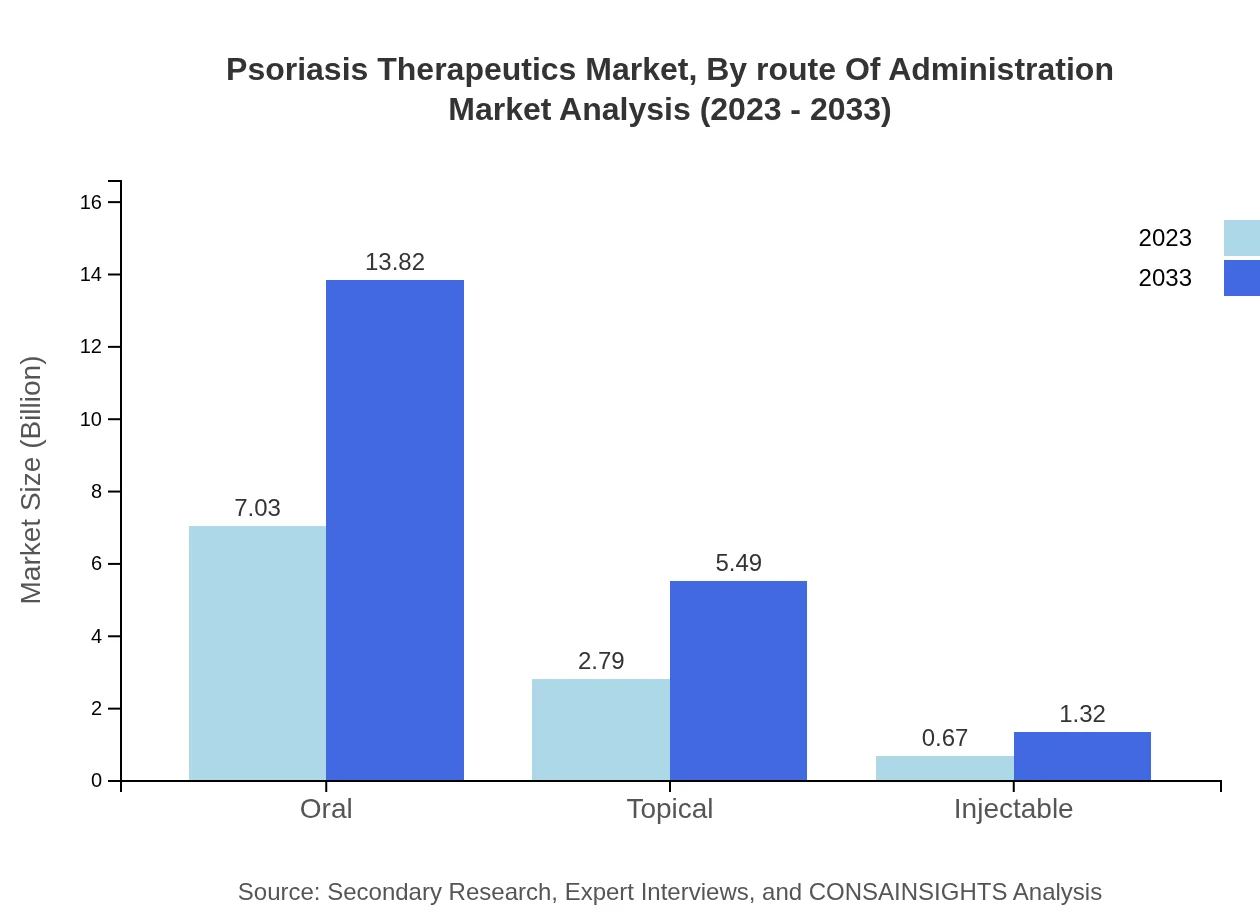
Psoriasis Therapeutics Market Analysis By Route Of Administration
The Oral route accounts for 66.99% of the market in 2023, valued at $7.03 billion, and is projected to grow significantly to $13.82 billion by 2033. This is followed by Topical and Injectable routes which account for 26.59% and 6.42% respectively, indicating a strong preference for convenient oral and topical therapies due to ease of administration.
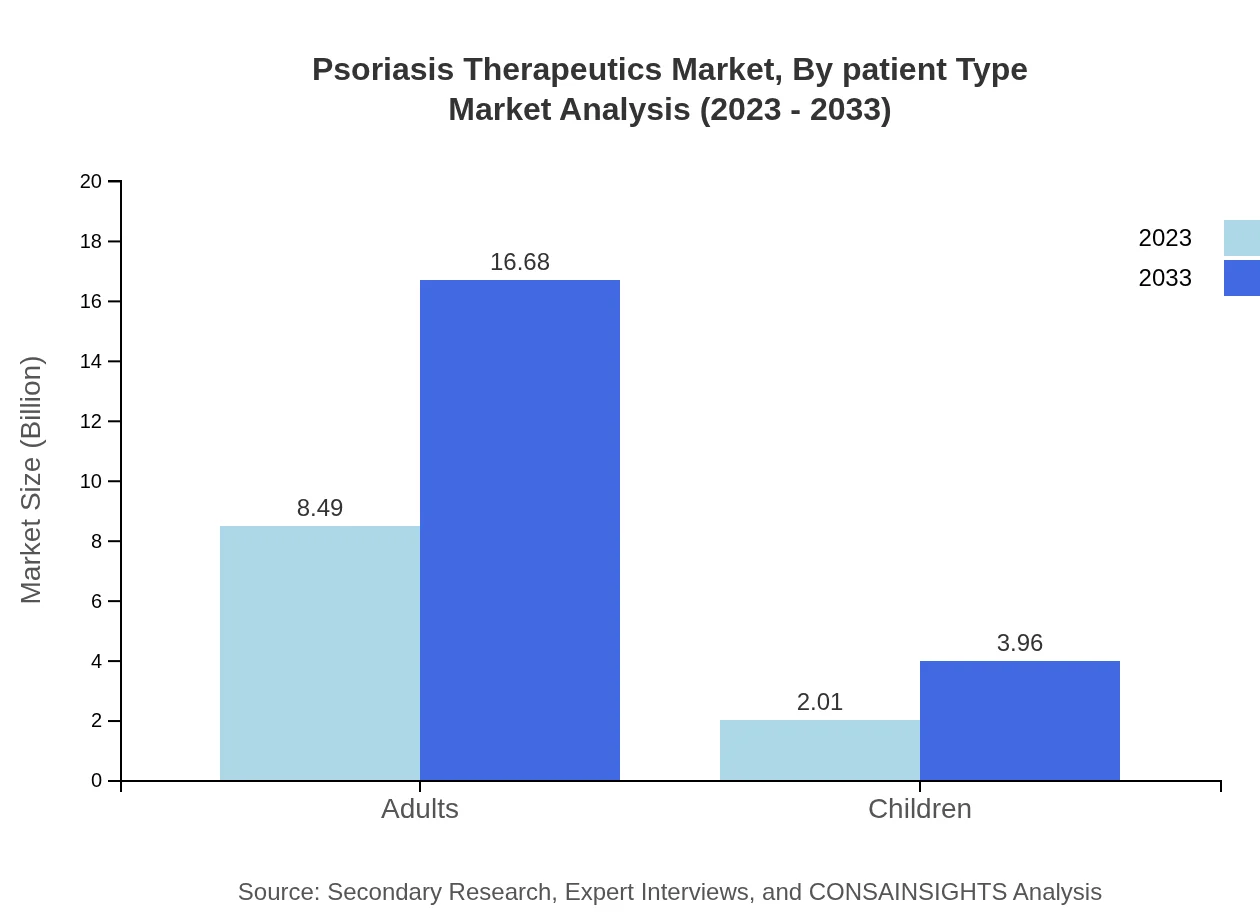
Psoriasis Therapeutics Market Analysis By Patient Type
The Adults segment dominates the market with a size of $8.49 billion in 2023, projected to grow to $16.68 billion by 2033. This segment makes up 80.83% of the total market share, while the Children segment, valued at $2.01 billion, is expected to increase to $3.96 billion, capturing 19.17% of the market share. Strategies targeting adults are likely essential for capturing the largest patient population.
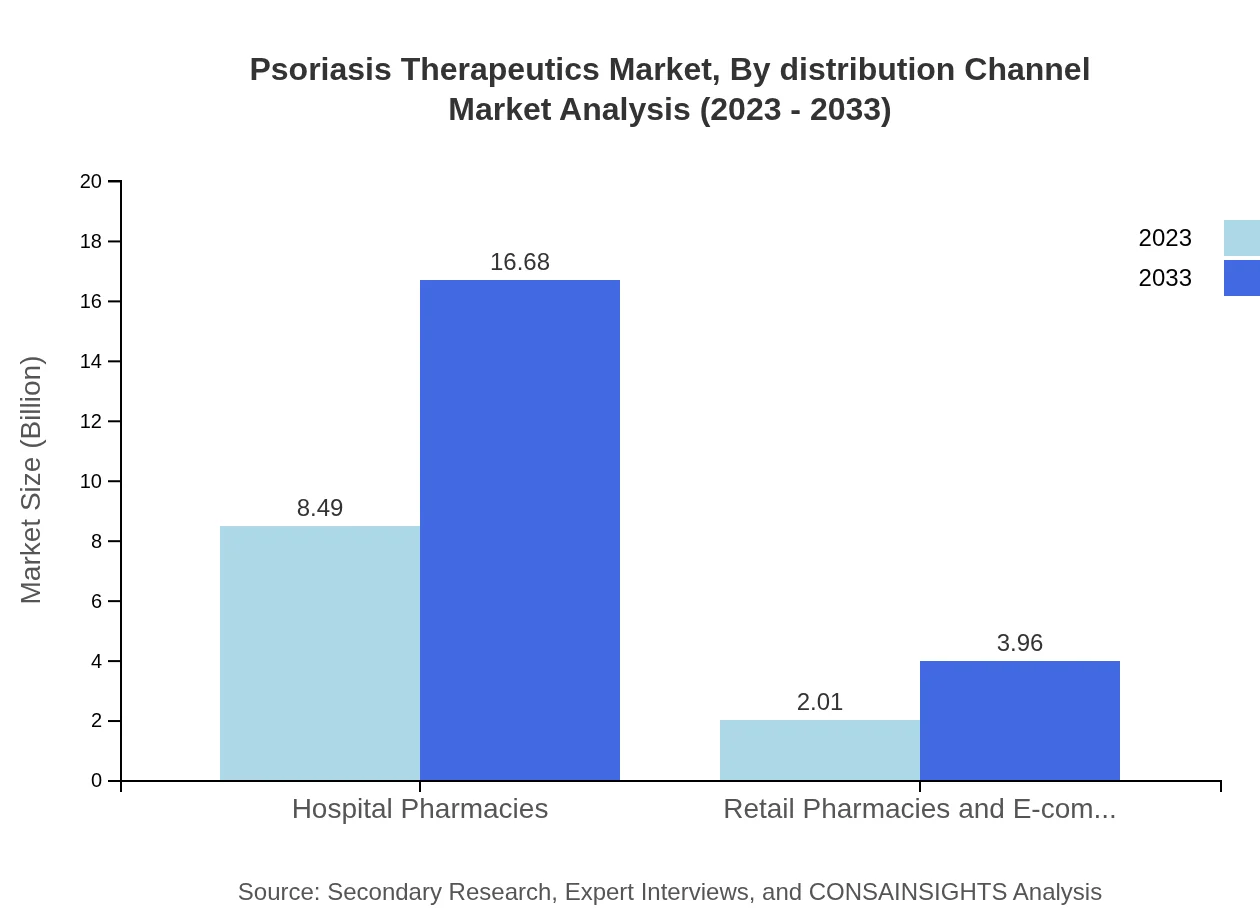
Psoriasis Therapeutics Market Analysis By Distribution Channel
Hospital Pharmacies lead with a market size of $8.49 billion in 2023, capturing 80.83% of the market share and forecasted to increase to $16.68 billion by 2033. Retail Pharmacies and E-commerce contribute a smaller share, valued at $2.01 billion (19.17% market share) in 2023 with projected growth to $3.96 billion, highlighting the importance of hospital settings in psoriasis management.
Psoriasis Therapeutics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Psoriasis Therapeutics Industry
AbbVie Inc.:
AbbVie is a leading biopharmaceutical company known for its extensive research and development in psoriasis treatment, particularly through its immunology portfolio including Humira and Rinvoq.Amgen Inc.:
Amgen is a biopharmaceutical company that focuses on innovative therapies in dermatology, having made significant strides in developing biologics for psoriasis management.Johnson & Johnson.:
Johnson & Johnson is a well-established healthcare company that provides a range of dermatological products, including those specifically designed for psoriasis, such as Tremfya.Novartis AG.:
Novartis is a global healthcare company recognized for its advancements in psoriasis therapies, including Cosentyx, which targets interleukin-17.We're grateful to work with incredible clients.









FAQs
What is the market size of psoriasis Therapeutics?
The global psoriasis therapeutics market is projected to reach $10.5 billion by 2033, growing at a CAGR of 6.8% from its current size. This significant market growth reflects the increasing awareness and demand for effective treatment solutions.
What are the key market players or companies in the psoriasis Therapeutics industry?
Key players in the psoriasis therapeutics market include major pharmaceutical companies such as AbbVie, Amgen, Johnson & Johnson, and Novartis. These companies are instrumental in developing innovative therapies and maintaining competitive advantages in this evolving market.
What are the primary factors driving the growth in the psoriasis Therapeutics industry?
Growth drivers for the psoriasis therapeutics market include rising incidence rates, increasing access to advanced treatments, heightened awareness among patients and healthcare professionals, and substantial investments in R&D for novel therapies, including biologics and systemic treatments.
Which region is the fastest Growing in the psoriasis Therapeutics market?
North America is the fastest-growing region in the psoriasis therapeutics market, expected to rise from $3.83 billion in 2023 to $7.53 billion by 2033. Europe and Asia Pacific also show significant growth, driven by increasing patient populations and healthcare spending.
Does ConsaInsights provide customized market report data for the psoriasis Therapeutics industry?
Yes, ConsaInsights offers customized market report data tailored to the specific needs of clients in the psoriasis therapeutics industry. This includes in-depth analysis of trends, forecasts, and competitive landscapes tailored to unique business objectives.
What deliverables can I expect from this psoriasis Therapeutics market research project?
Deliverables from the psoriasis therapeutics market research project include comprehensive market analysis reports, forecast data, competitive profiling, regional insights, and strategic recommendations, all aimed at informing business decisions and market strategies.
What are the market trends of psoriasis Therapeutics?
Current market trends in psoriasis therapeutics include an increasing shift toward biologic therapies, growing preferences for personalized medicine, advancements in telemedicine, and a heightened focus on patient-centric treatment approaches within the healthcare system.