Psoriatic Arthritis Therapeutics Market Report
Published Date: 31 January 2026 | Report Code: psoriatic-arthritis-therapeutics
Psoriatic Arthritis Therapeutics Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Psoriatic Arthritis Therapeutics market, showcasing market size, trends, and forecasts from 2023 to 2033, along with a detailed segmentation, regional insights, and key players in the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
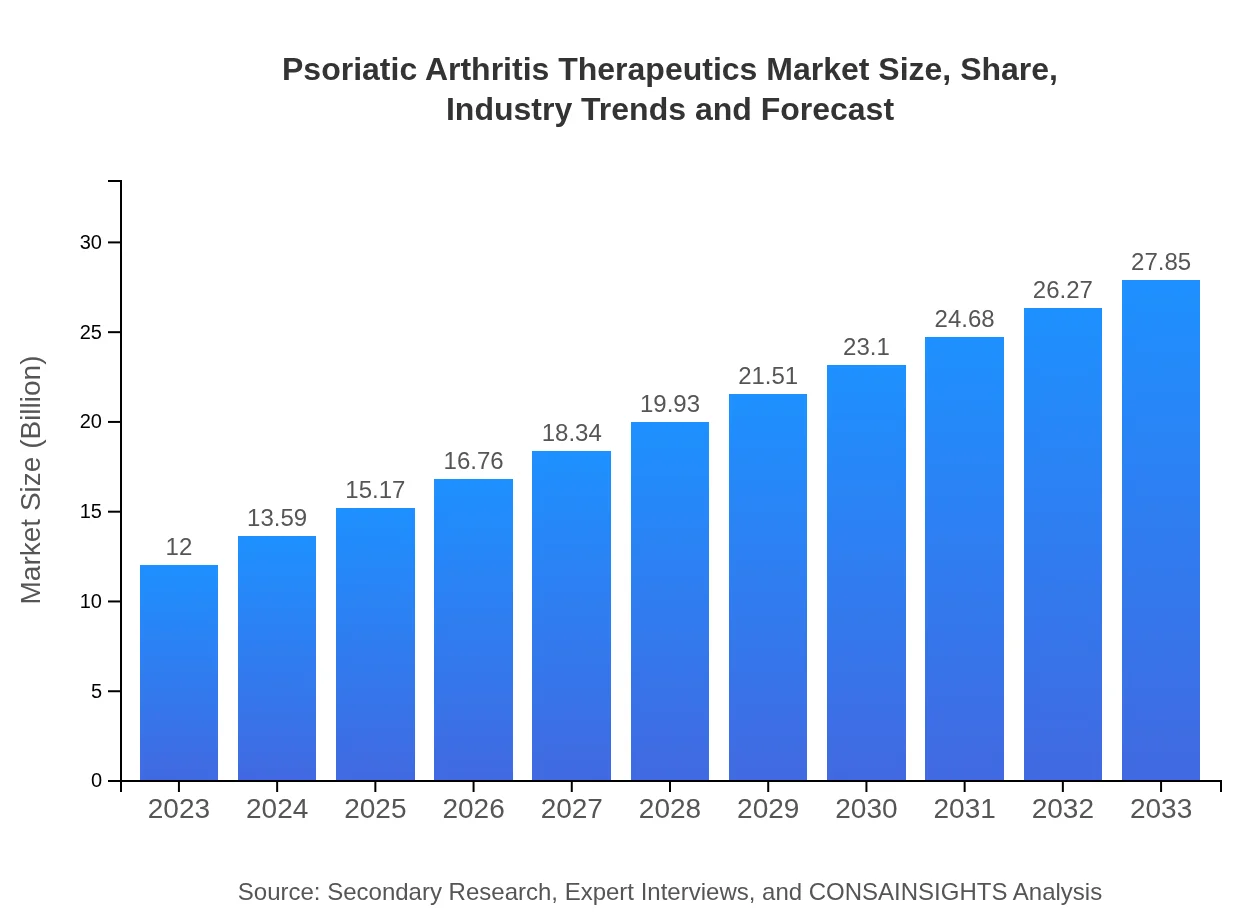
| 2023 Market Size | $12.00 Billion |
| CAGR (2023-2033) | 8.5% |
| 2033 Market Size | $27.85 Billion |
| Top Companies | AbbVie, Johnson & Johnson, Amgen, Novartis |
| Last Modified Date | 31 January 2026 |
Psoriatic Arthritis Therapeutics Market Overview
Customize Psoriatic Arthritis Therapeutics Market Report market research report
- ✔ Get in-depth analysis of Psoriatic Arthritis Therapeutics market size, growth, and forecasts.
- ✔ Understand Psoriatic Arthritis Therapeutics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Psoriatic Arthritis Therapeutics
What is the Market Size & CAGR of the Psoriatic Arthritis Therapeutics market in 2023?
Psoriatic Arthritis Therapeutics Industry Analysis
Psoriatic Arthritis Therapeutics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Psoriatic Arthritis Therapeutics Market Analysis Report by Region
Europe Psoriatic Arthritis Therapeutics Market Report:
The European market for Psoriatic Arthritis therapeutics is poised for significant growth, from $3.19 billion in 2023 to $7.41 billion in 2033. The market is supported by a well-established reimbursement framework and ongoing R&D efforts by pharmaceutical companies.Asia Pacific Psoriatic Arthritis Therapeutics Market Report:
The Asia Pacific market for PsA therapeutics is valued at approximately $2.35 billion in 2023 and is expected to reach $5.45 billion by 2033. Significant growth is driven by increasing patient awareness and access to healthcare services, along with rising healthcare expenditure in emerging economies.North America Psoriatic Arthritis Therapeutics Market Report:
In North America, the PsA therapeutics market is expected to reach $10.78 billion by 2033, growing from $4.65 billion in 2023. The primary drivers include a high prevalence of psoriatic arthritis, extensive research activities, and favorable reimbursement policies.South America Psoriatic Arthritis Therapeutics Market Report:
The South American market is projected to grow from $1.17 billion in 2023 to $2.72 billion by 2033. Factors such as improvements in healthcare infrastructure and increased availability of novel therapies are expected to fuel market growth.Middle East & Africa Psoriatic Arthritis Therapeutics Market Report:
The Middle East and Africa market is projected to increase from $0.64 billion in 2023 to $1.48 billion by 2033, led by rising healthcare investments and increasing awareness programs regarding psoriatic arthritis treatments.Tell us your focus area and get a customized research report.
Psoriatic Arthritis Therapeutics Market Analysis By Drug Class
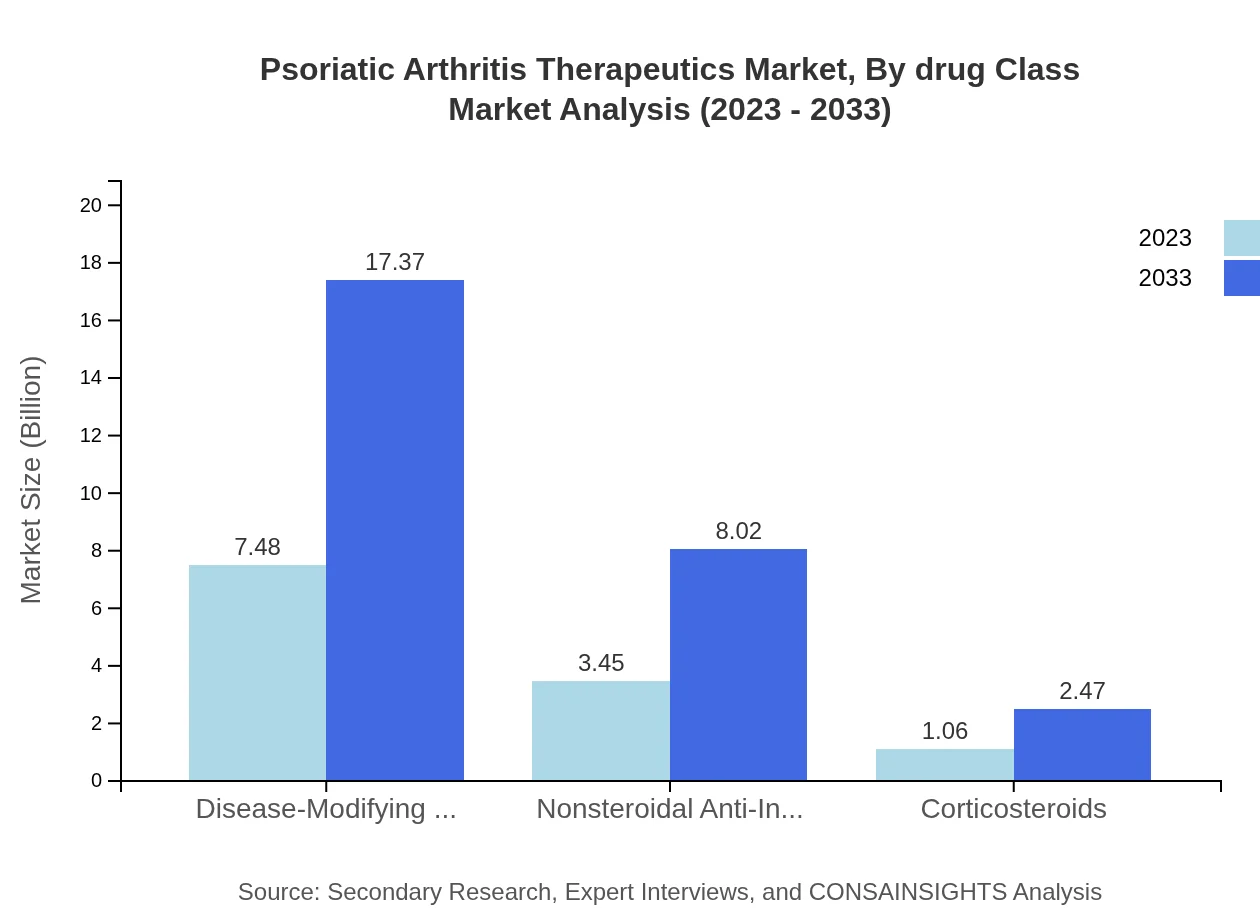
In 2023, the market for Disease-Modifying Antirheumatic Drugs (DMARDs) is valued at $7.48 billion, holding a 62.35% share. This segment is projected to reach $17.37 billion by 2033, reflecting the efficacy of DMARDs in providing long-term relief and managing disease progression. Non-steroidal Anti-Inflammatory Drugs (NSAIDs) will reach $8.02 billion by 2033 from $3.45 billion in 2023, maintaining a steady share of 28.78%. Corticosteroids, while smaller with $1.06 billion in 2023, are expected to rise to $2.47 billion, representing 8.87% by 2033.
Psoriatic Arthritis Therapeutics Market Analysis By Route Of Administration
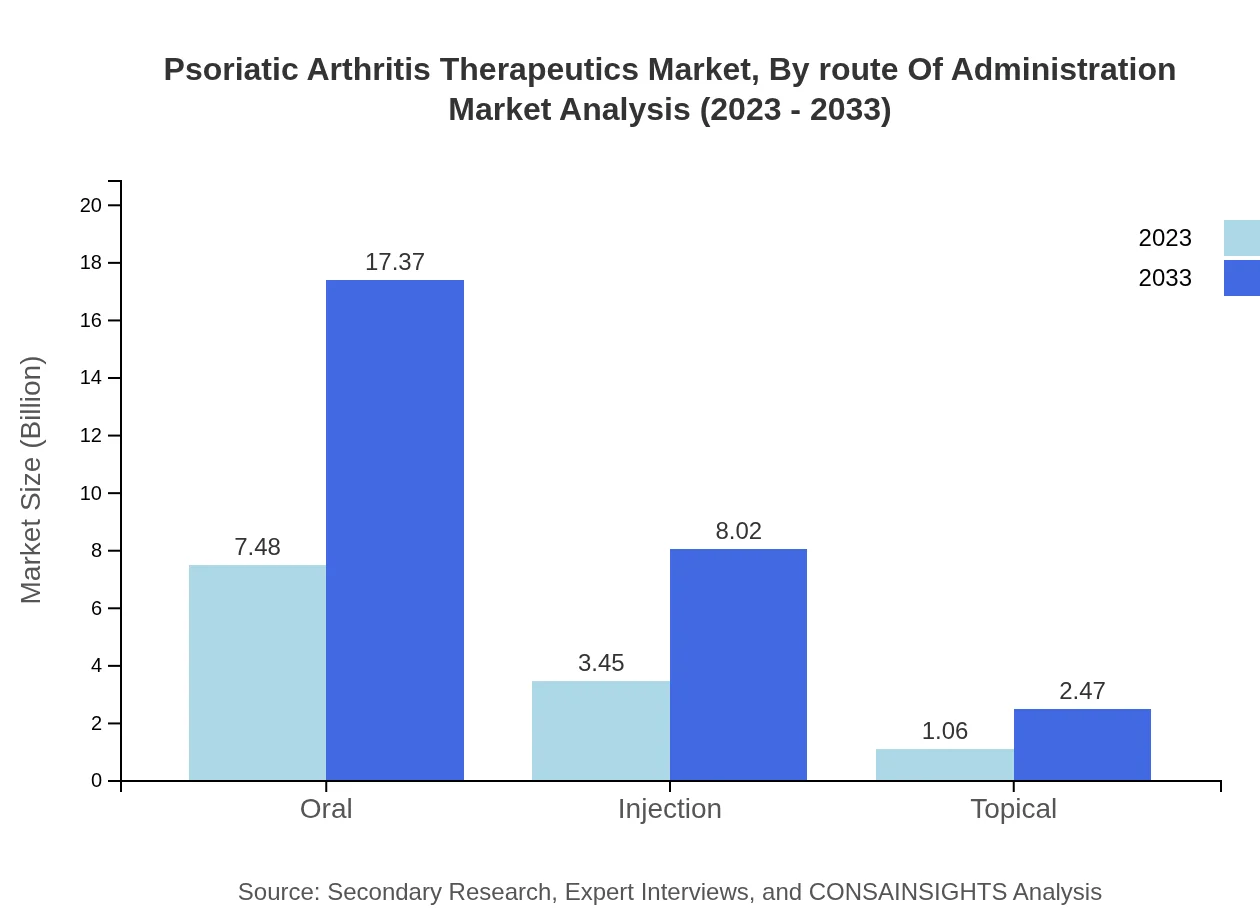
The oral route dominates with $7.48 billion in 2023 and a share of 62.35%, while injections and topical formulations contribute $3.45 billion and $1.06 billion, respectively. By 2033, oral administration will maintain its leading position, projected at $17.37 billion, while injections and topical revenues are anticipated at $8.02 billion and $2.47 billion.
Psoriatic Arthritis Therapeutics Market Analysis By Patient Type
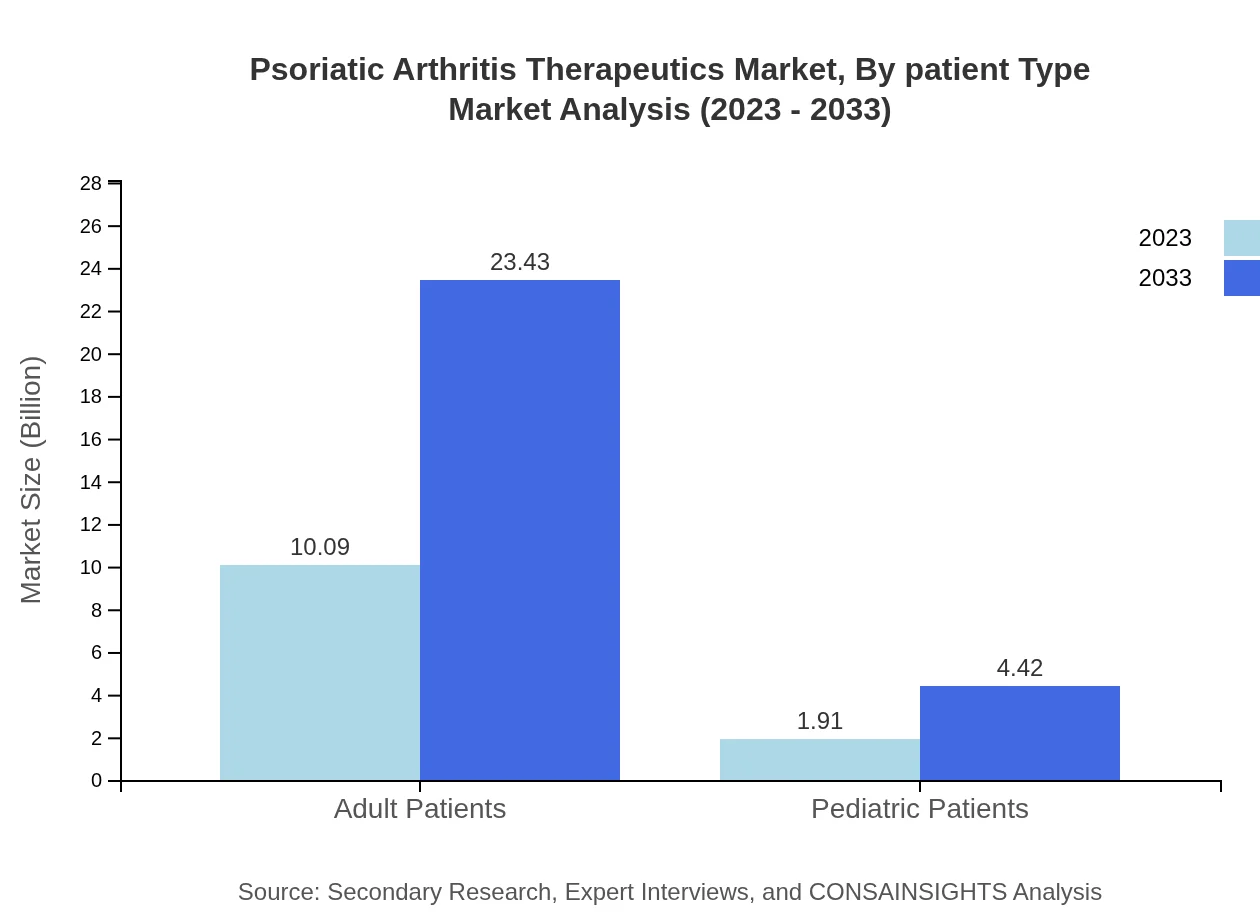
For adult patients, the market is currently valued at $10.09 billion and is projected to reach $23.43 billion by 2033, capturing an overwhelming 84.12% of the market. Pediatric patients, currently at $1.91 billion, are expected to grow to $4.42 billion, making up 15.88% of the market share.
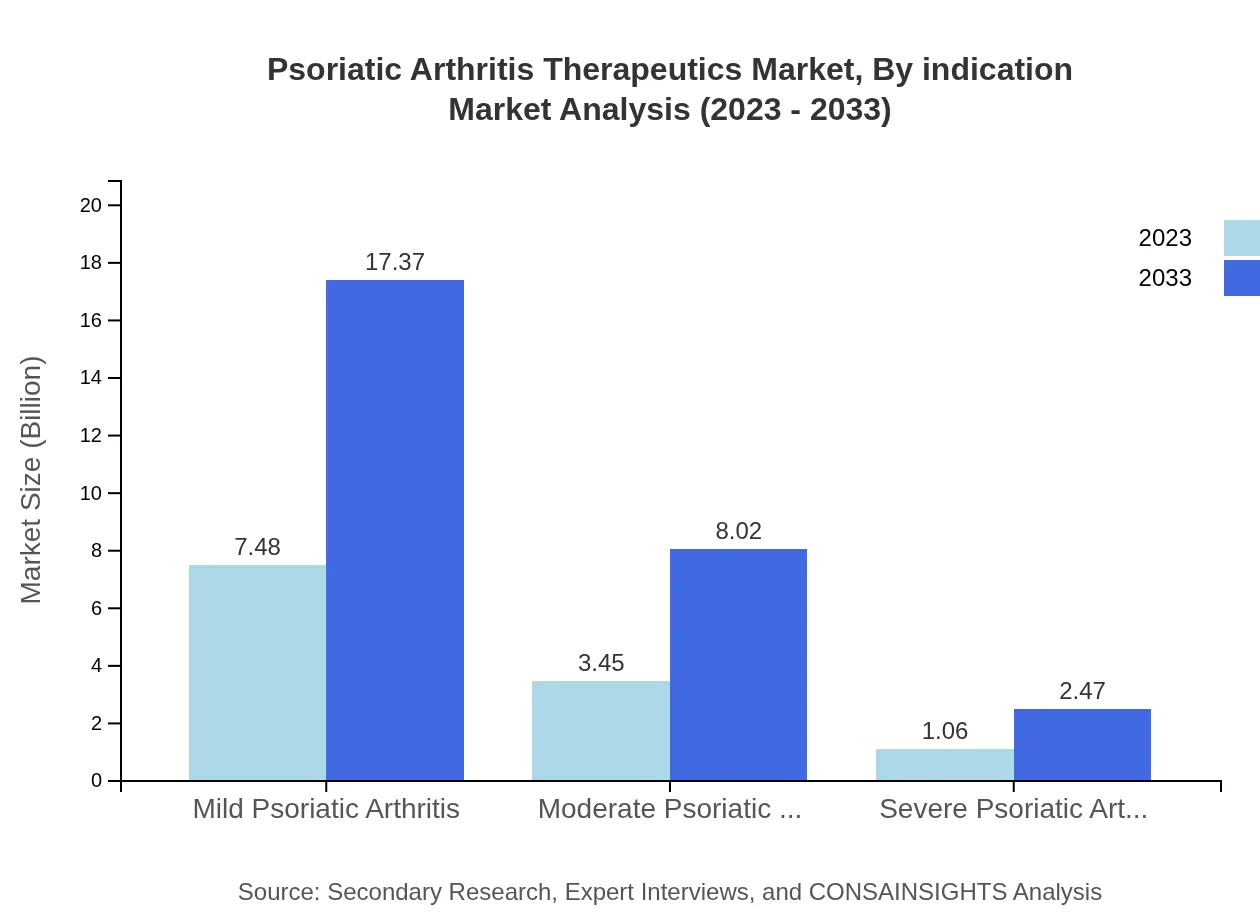
Psoriatic Arthritis Therapeutics Market Analysis By Indication
The market for mild psoriatic arthritis currently accounts for $7.48 billion and is expected to grow exponentially to $17.37 billion by 2033, sharing 62.35%. Moderate and severe cases are projected to reach $8.02 billion and $2.47 billion, respectively, indicating the need for targeted therapies as disease severity increases.
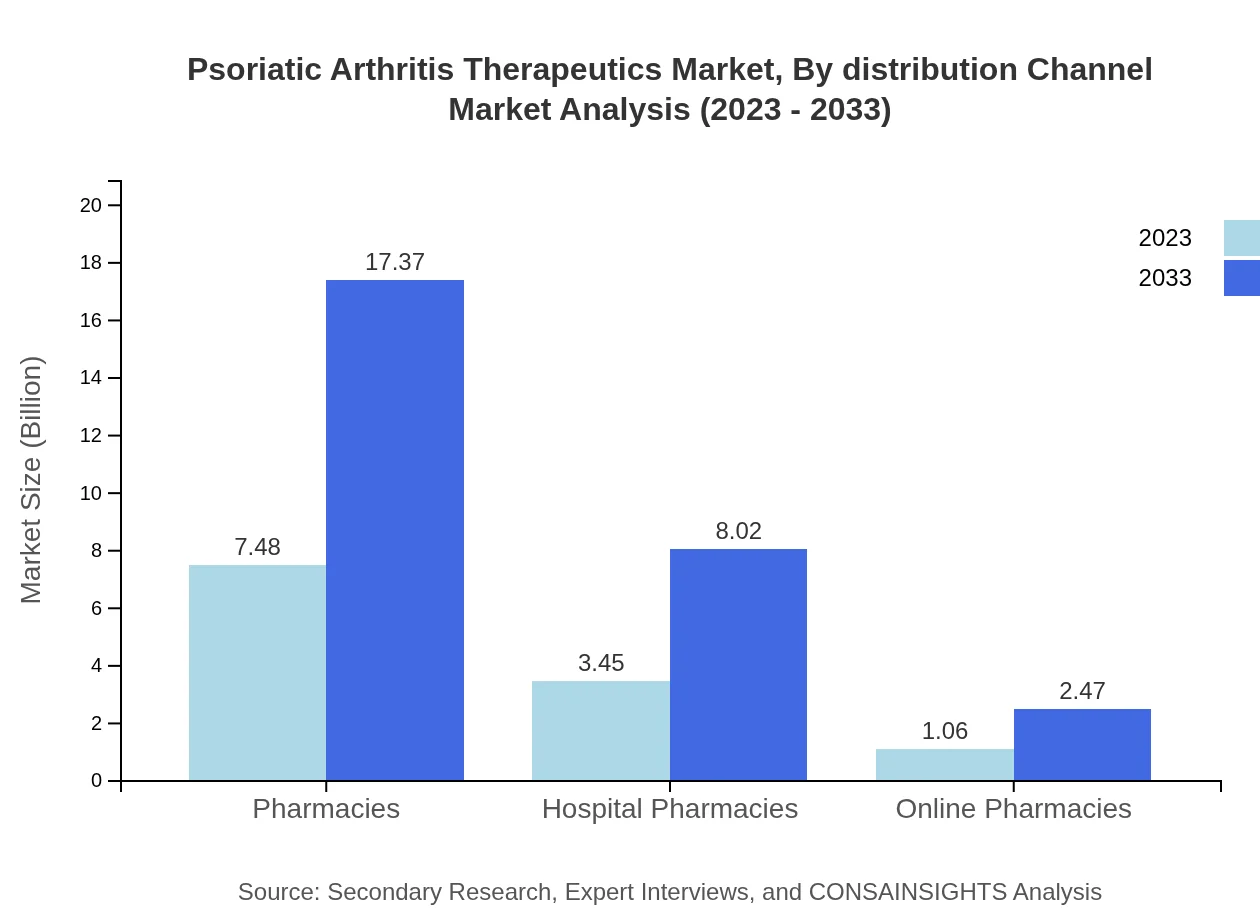
Psoriatic Arthritis Therapeutics Market Analysis By Distribution Channel
Retail pharmacies, holding a size of $7.48 billion (62.35% share) in 2023, are expected to grow to $17.37 billion by 2033. Hospital pharmacies and online pharmacies will also capture significant market segments at $8.02 billion and $2.47 billion respectively, by that year.
Psoriatic Arthritis Therapeutics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Psoriatic Arthritis Therapeutics Industry
AbbVie:
AbbVie is a global biopharmaceutical company leading in research and development of innovative treatments for psoriatic arthritis, particularly with drugs like Humira and Rinvoq.Johnson & Johnson:
Johnson & Johnson focuses on developing comprehensive therapeutics for autoimmune diseases, with drugs like Tremfya addressing psoriatic arthritis.Amgen:
Amgen is known for its advanced biologics including Enbrel, a key treatment for psoriatic arthritis, enhancing patient outcomes.Novartis:
Novartis provides a range of innovative medications targeting various aspects of psoriatic arthritis, including Cosentyx, which has significantly improved therapy options.We're grateful to work with incredible clients.









FAQs
What is the market size of psoriatic arthritis therapeutics?
The psoriatic arthritis therapeutics market size is projected to reach approximately $12 billion by 2033, with a robust CAGR of 8.5%. This growth reflects increasing demand for effective therapies and an expanding patient population.
What are the key market players or companies in the psoriatic arthritis therapeutics industry?
Key players in the psoriatic arthritis therapeutics market include pharmaceutical companies such as Amgen, AbbVie, Johnson & Johnson, Pfizer, and UCB. These companies are at the forefront, developing innovations and treatments for psoriatic arthritis.
What are the primary factors driving the growth in the psoriatic arthritis therapeutics industry?
The primary drivers of growth in the psoriatic arthritis therapeutics market include an increase in prevalence rates, advancements in biologic therapies, growing awareness among healthcare providers, and an expanding pool of diagnosed patients requiring effective treatments.
Which region is the fastest Growing in the psoriatic arthritis therapeutics market?
The fastest-growing region in the psoriatic arthritis therapeutics market is expected to be North America, with projections indicating growth from $4.65 billion in 2023 to $10.78 billion by 2033, driven by rising incidence and healthcare advancements.
Does ConsaInsights provide customized market report data for the psoriatic arthritis therapeutics industry?
Yes, ConsaInsights offers customized market report data tailored to the specific needs of clients within the psoriatic arthritis therapeutics industry, ensuring relevant insights and strategic advantages for decision-making.
What deliverables can I expect from this psoriatic arthritis therapeutics market research project?
Clients can expect comprehensive deliverables including detailed market analysis, segmentation insights, competitor profiling, detailed trends, forecasts, and tailored recommendations to effectively navigate the psoriatic arthritis therapeutics landscape.
What are the market trends of psoriatic arthritis therapeutics?
Market trends indicate a shift towards personalized medicine, increased adoption of biologics and systemic therapies, greater focus on patient-centric care, and a rise in telehealth solutions, which together foster improved management of psoriatic arthritis.