Pulmonary Drug Delivery Devices Market Report
Published Date: 31 January 2026 | Report Code: pulmonary-drug-delivery-devices
Pulmonary Drug Delivery Devices Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Pulmonary Drug Delivery Devices market, covering insights into market size, trends, technology, and growth forecasts for the period 2023-2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
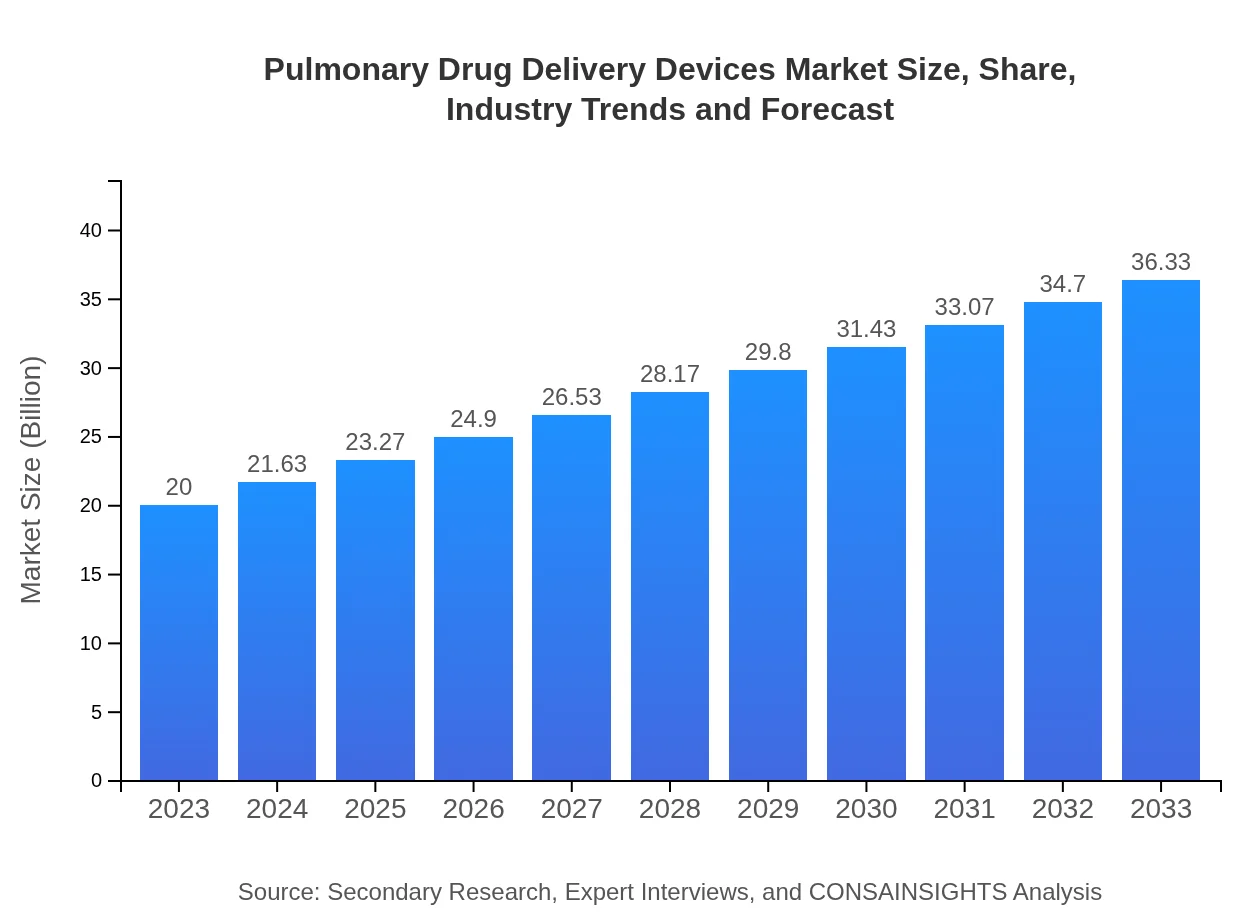
| 2023 Market Size | $20.00 Billion |
| CAGR (2023-2033) | 6% |
| 2033 Market Size | $36.33 Billion |
| Top Companies | AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Teva Pharmaceuticals |
| Last Modified Date | 31 January 2026 |
Pulmonary Drug Delivery Devices Market Overview
Customize Pulmonary Drug Delivery Devices Market Report market research report
- ✔ Get in-depth analysis of Pulmonary Drug Delivery Devices market size, growth, and forecasts.
- ✔ Understand Pulmonary Drug Delivery Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Pulmonary Drug Delivery Devices
What is the Market Size & CAGR of Pulmonary Drug Delivery Devices market in 2023?
Pulmonary Drug Delivery Devices Industry Analysis
Pulmonary Drug Delivery Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Pulmonary Drug Delivery Devices Market Analysis Report by Region
Europe Pulmonary Drug Delivery Devices Market Report:
Europe's market for Pulmonary Drug Delivery Devices is anticipated to rise to $10.01 billion by 2033, significantly increasing from $5.51 billion in 2023. An aging population, coupled with increased government spending on healthcare, shapes the market's growth trajectory.Asia Pacific Pulmonary Drug Delivery Devices Market Report:
The Asia Pacific region is projected to experience significant growth in the Pulmonary Drug Delivery Devices market, driven by rising air pollution levels and the increasing prevalence of respiratory diseases. By 2033, the market is expected to reach approximately $7.22 billion, up from $3.97 billion in 2023, reflecting a robust CAGR that underscores the region's growing healthcare demands.North America Pulmonary Drug Delivery Devices Market Report:
North America remains a dominant player in the Pulmonary Drug Delivery Devices market, with a projected market size of $12.67 billion by 2033, growing from $6.98 billion in 2023. This growth is attributed to advanced healthcare infrastructure and a strong emphasis on chronic disease management.South America Pulmonary Drug Delivery Devices Market Report:
In South America, the Pulmonary Drug Delivery Devices market is expected to grow steadily, reaching $3.35 billion by 2033 from $1.84 billion in 2023. Increasing healthcare expenditures and a focus on improving respiratory care access will drive market expansion in this region.Middle East & Africa Pulmonary Drug Delivery Devices Market Report:
In the Middle East and Africa, the market is forecasted to expand reaching $3.08 billion by 2033 from $1.70 billion in 2023. Growth drivers include legislative support for healthcare innovations and rising awareness of pulmonary care.Tell us your focus area and get a customized research report.
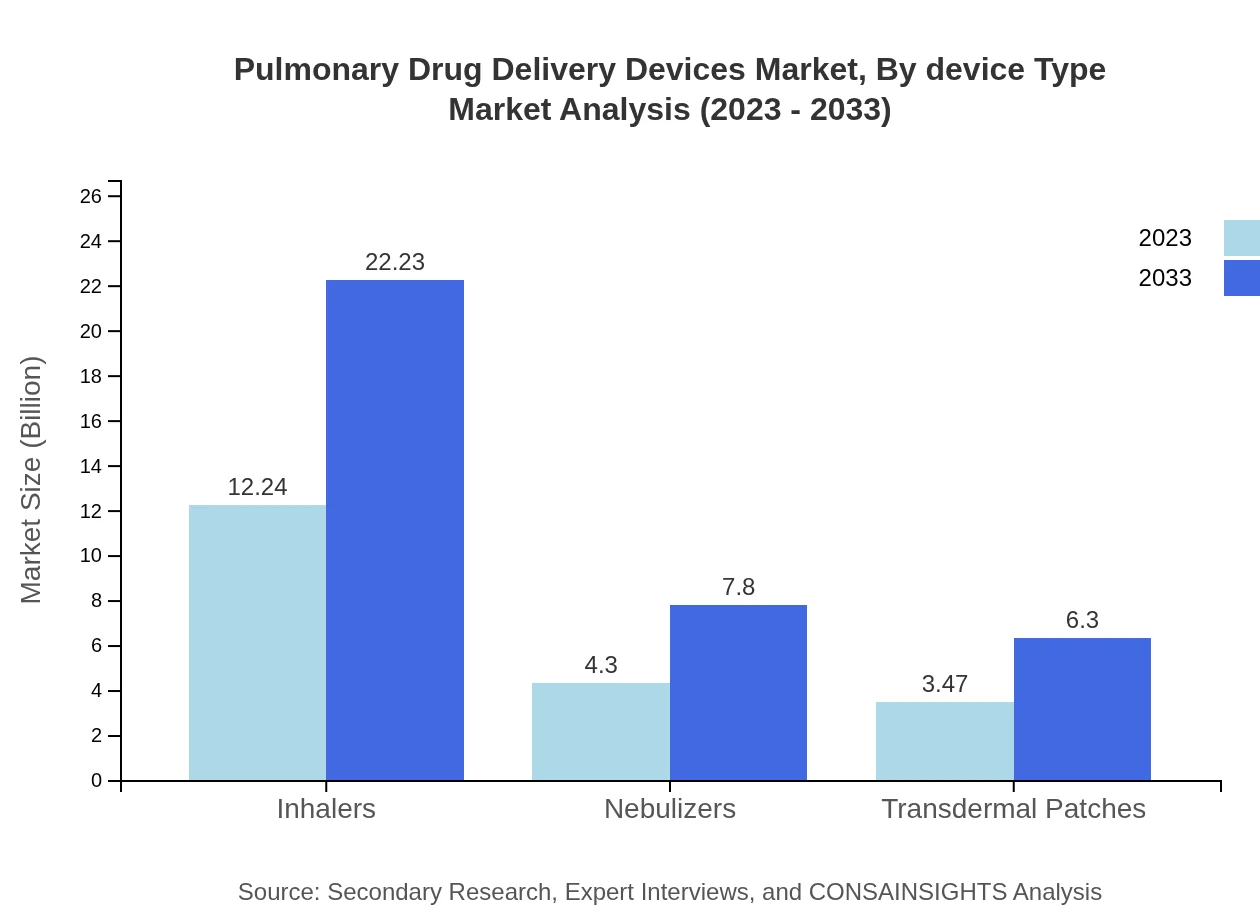
Pulmonary Drug Delivery Devices Market Analysis By Device Type
In 2023, inhalers account for a notable market share, valued at $12.24 billion and expected to grow to $22.23 billion by 2033. This segment leads the market due to its convenience and widespread acceptance among users. Following inhalers are nebulizers, with a size of $4.30 billion in 2023, expected to reach $7.80 billion by 2033. Transdermal patches, while smaller, show promise, with a projected increase from $3.47 billion in 2023 to $6.30 billion by 2033.
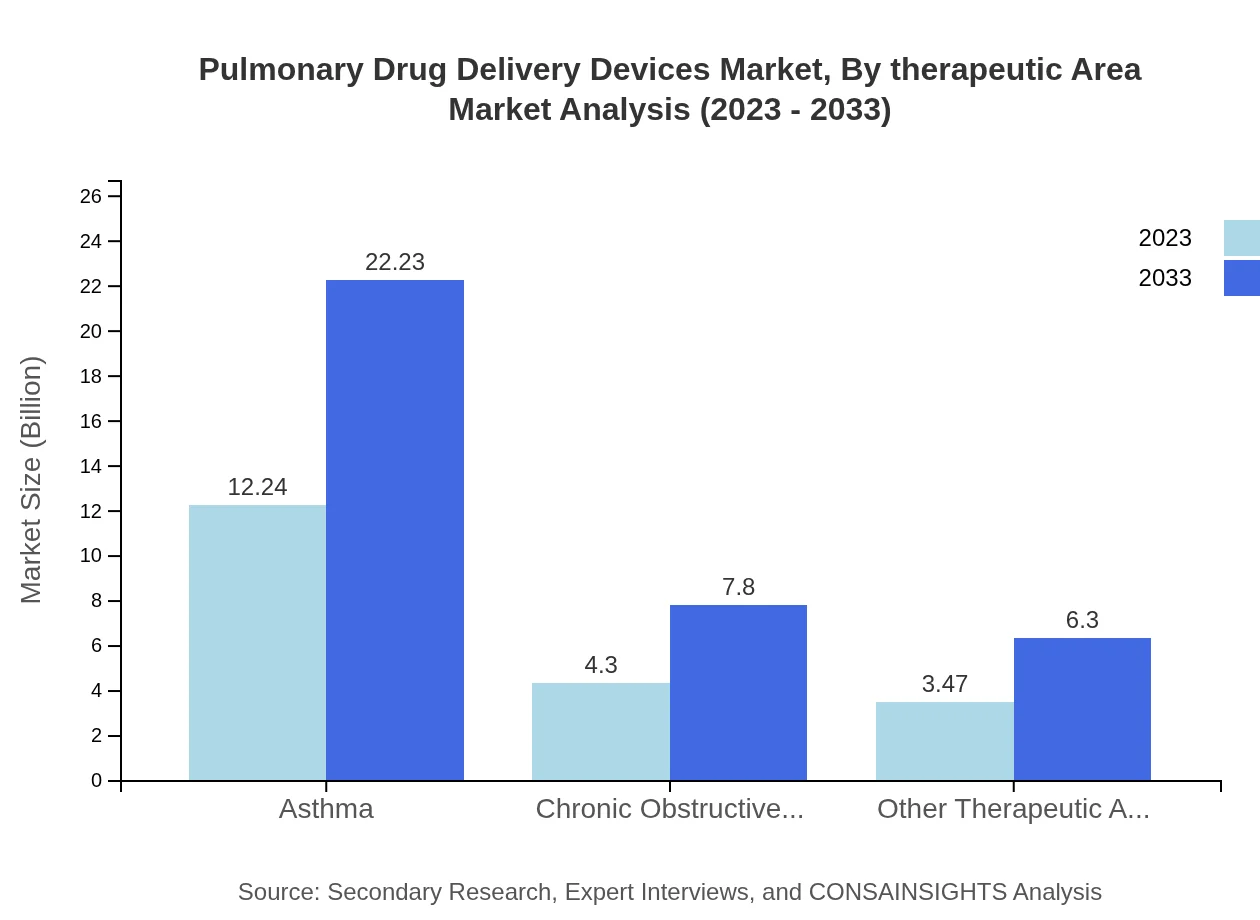
Pulmonary Drug Delivery Devices Market Analysis By Therapeutic Area
Asthma remains the leading therapeutic area for pulmonary drug delivery, with a market size of $12.24 billion in 2023 and anticipated growth to $22.23 billion by 2033. Chronic Obstructive Pulmonary Disease (COPD) follows, with an increase from $4.30 billion to $7.80 billion in the same period. Other therapeutic areas, including various pulmonary health concerns, highlight the increasing diversification in patient treatment options.
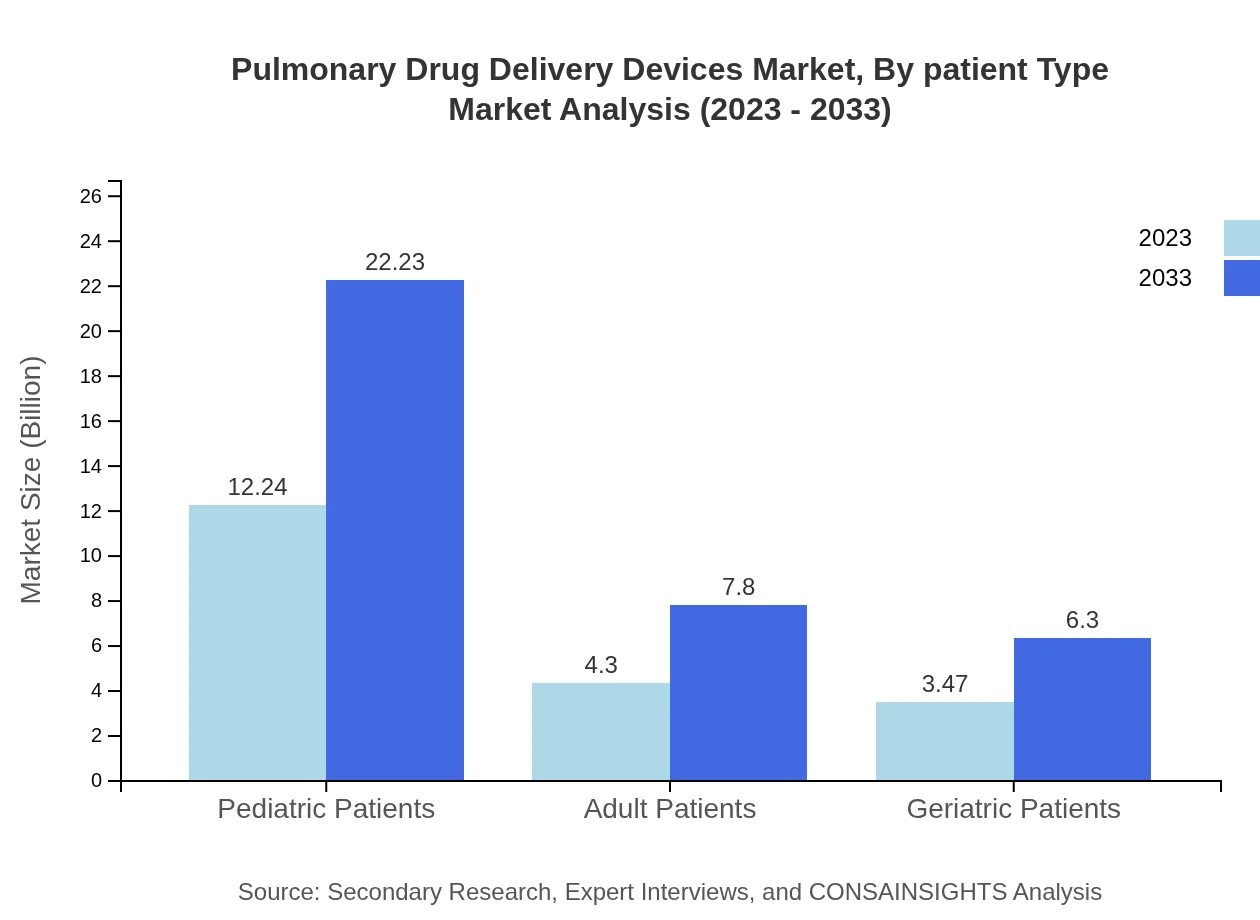
Pulmonary Drug Delivery Devices Market Analysis By Patient Type
The market for pulmonary drug delivery devices emphasizes pediatric patients, representing a significant share with a size of $12.24 billion in 2023 and predicted to reach $22.23 billion by 2033. Adult patients constitute a smaller segment, growing from $4.30 billion to $7.80 billion, followed by geriatric patients showing steady growth from $3.47 billion to $6.30 billion.
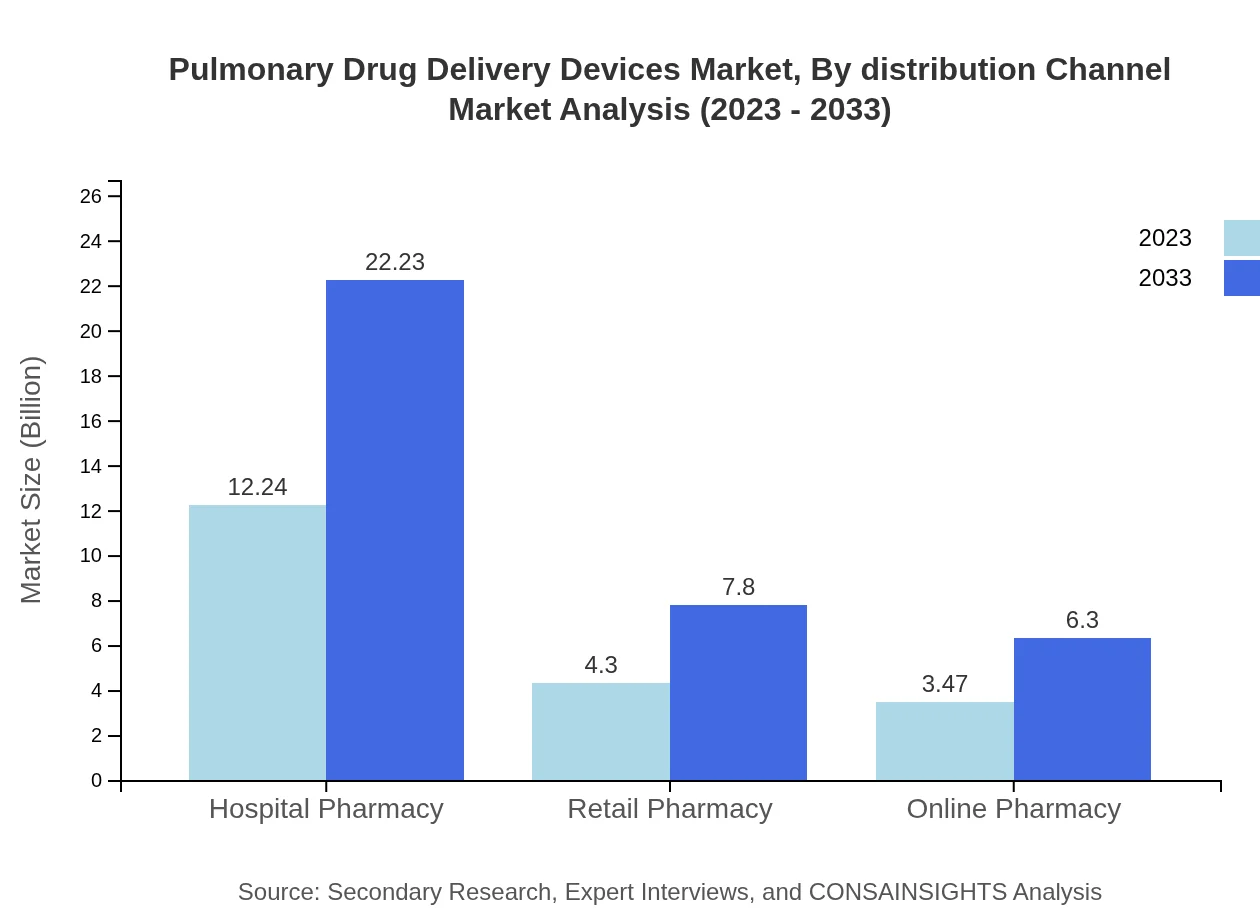
Pulmonary Drug Delivery Devices Market Analysis By Distribution Channel
Hospital pharmacies represent the largest distribution channel, valued at $12.24 billion in 2023, set to increase to $22.23 billion by 2033. Retail pharmacies hold a substantial market share, growing from $4.30 billion to $7.80 billion, while online pharmacies are emerging as a competitive channel with expected growth from $3.47 billion to $6.30 billion.
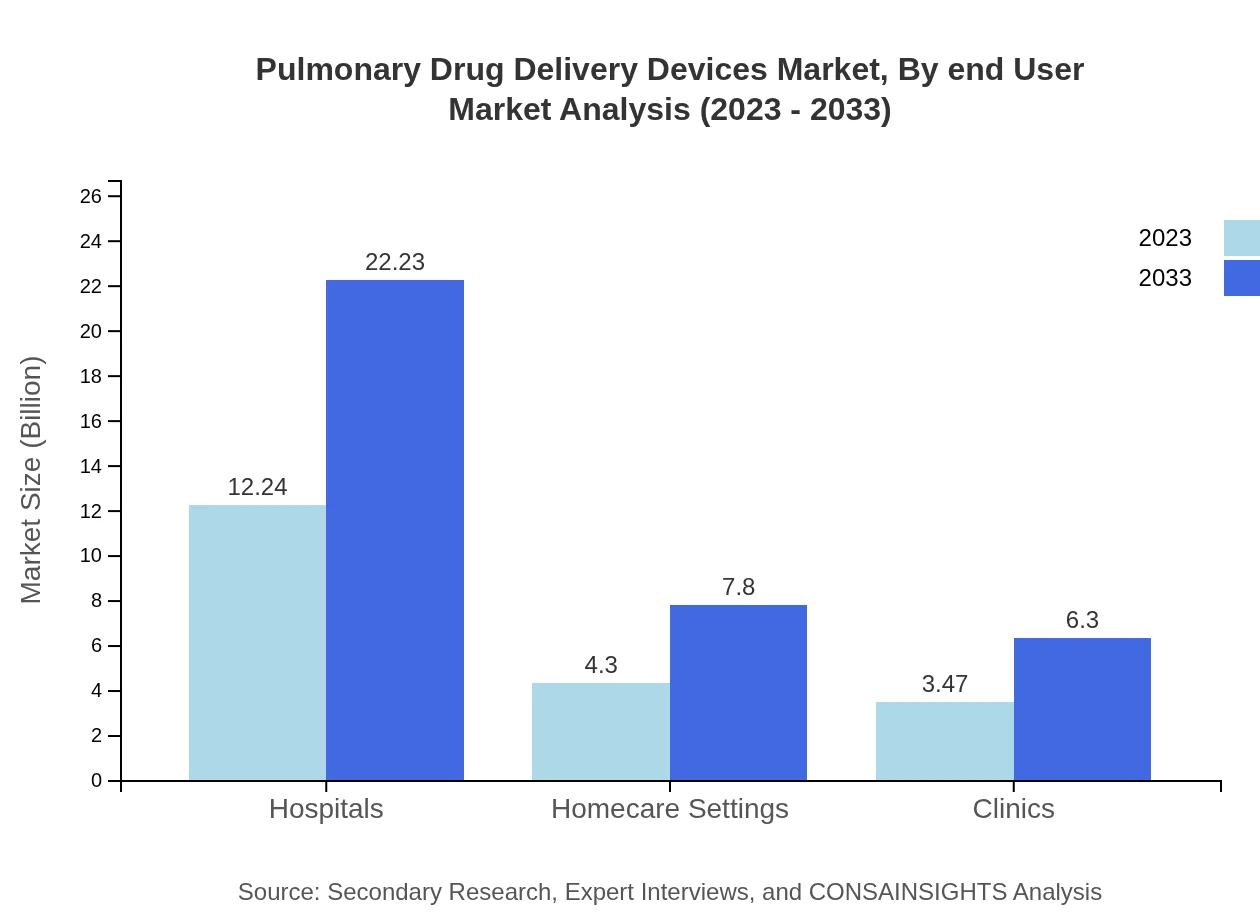
Pulmonary Drug Delivery Devices Market Analysis By End User
Demand for pulmonary drug delivery devices is seeing growth across various end-users, particularly in hospitals, which hold the largest market share expected to reach $22.23 billion by 2033 from $12.24 billion in 2023. Clinics and homecare settings diversify the market, with respective projections of growth reflecting the growing preference for outpatient care.
Pulmonary Drug Delivery Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Pulmonary Drug Delivery Devices Industry
AstraZeneca:
AstraZeneca is a leading global biopharmaceutical company known for its advanced development of inhalers for asthma and COPD, significantly contributing to the pulmonary drug delivery devices market.Boehringer Ingelheim:
Boehringer Ingelheim specializes in respiratory treatment solutions, with its portfolio of nebulizers and inhalers aimed at improving patient outcomes in lung health.GlaxoSmithKline:
GlaxoSmithKline is a prominent player in the pulmonary drug delivery market, known for its innovative inhalation therapies that address a wide range of respiratory diseases.Teva Pharmaceuticals:
Teva is a leading generic pharmaceuticals company that offers a variety of inhalation devices and medications targeted at both COPD and asthma management.We're grateful to work with incredible clients.









FAQs
What is the market size of pulmonary Drug Delivery Devices?
The Pulmonary Drug Delivery Devices market is valued at approximately $20 billion in 2023, with an expected compound annual growth rate (CAGR) of 6%, projected to reach significant growth by 2033.
What are the key market players or companies in this pulmonary Drug Delivery Devices industry?
Key players in the Pulmonary Drug Delivery Devices industry include major pharmaceutical companies, medical device manufacturers, and biotech firms focusing on innovative inhalation therapies and drug delivery systems.
What are the primary factors driving the growth in the pulmonary Drug Delivery Devices industry?
Factors driving market growth include increasing prevalence of respiratory diseases, rising demand for advanced drug delivery systems, and technological advancements enhancing device efficiency and patient compliance.
Which region is the fastest Growing in the pulmonary Drug Delivery Devices?
The Asia Pacific region is the fastest-growing in the Pulmonary Drug Delivery Devices market, with expected growth from $3.97 billion in 2023 to $7.22 billion by 2033.
Does ConsaInsights provide customized market report data for the pulmonary Drug Delivery Devices industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the pulmonary drug delivery devices industry, accommodating various aspects of market analysis.
What deliverables can I expect from this pulmonary Drug Delivery Devices market research project?
Deliverables from the Pulmonary Drug Delivery Devices market research project include detailed market size analysis, growth forecasts, competitive landscape evaluations, and insights into market trends and consumer behavior.
What are the market trends of pulmonary Drug Delivery Devices?
Market trends in pulmonary drug delivery devices highlight a shift towards personalized medicine, increased adoption of digital health solutions, and ongoing innovation in delivery technologies for improved patient outcomes.