Pulmonary Drug Delivery Systems Market Report
Published Date: 31 January 2026 | Report Code: pulmonary-drug-delivery-systems
Pulmonary Drug Delivery Systems Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Pulmonary Drug Delivery Systems market, highlighting key market trends, regional insights, and growth forecasts from 2023 to 2033, offering valuable insights for stakeholders and industry players.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
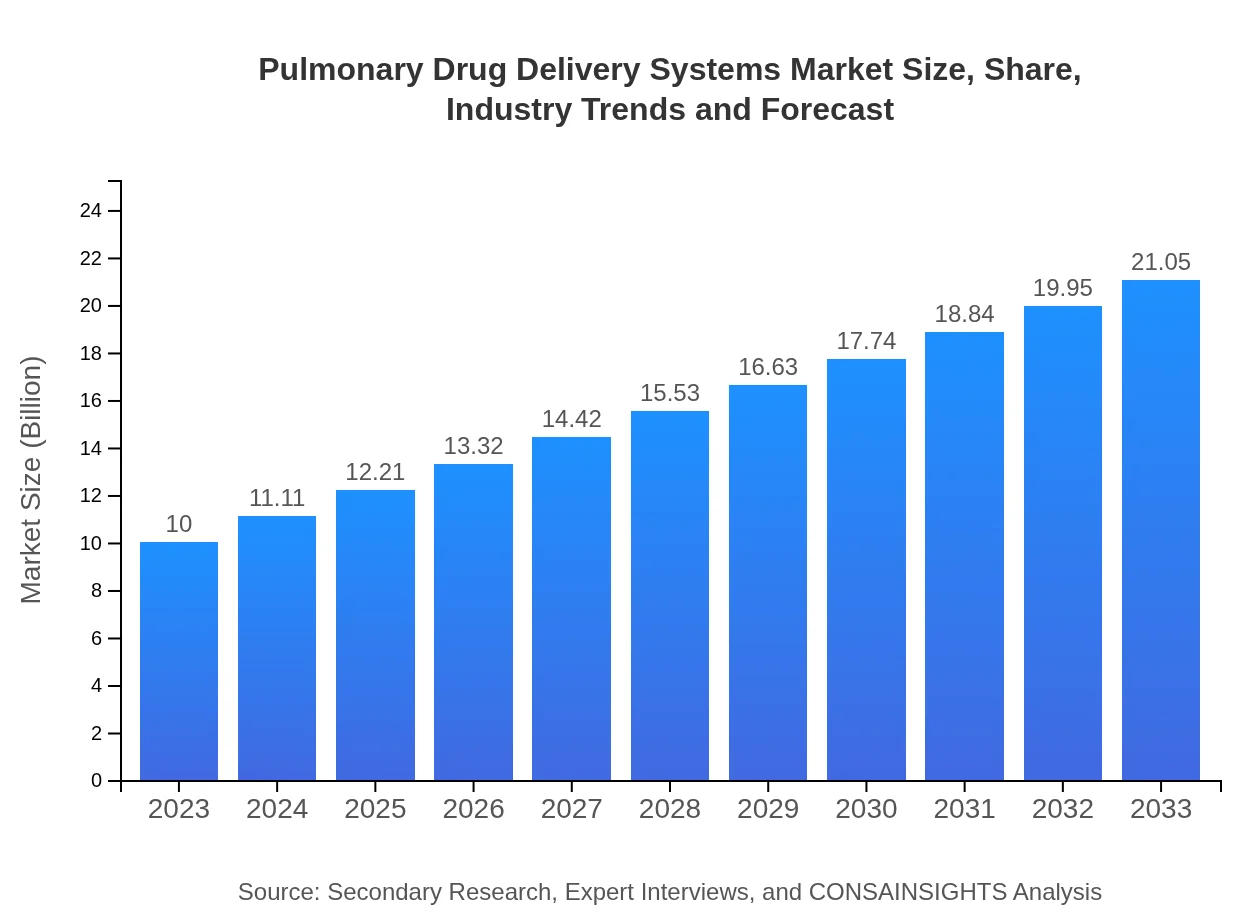
| 2023 Market Size | $10.00 Billion |
| CAGR (2023-2033) | 7.5% |
| 2033 Market Size | $21.05 Billion |
| Top Companies | AstraZeneca, GlaxoSmithKline, Novartis, Boehringer Ingelheim, Teva Pharmaceuticals |
| Last Modified Date | 31 January 2026 |
Pulmonary Drug Delivery Systems Market Overview
Customize Pulmonary Drug Delivery Systems Market Report market research report
- ✔ Get in-depth analysis of Pulmonary Drug Delivery Systems market size, growth, and forecasts.
- ✔ Understand Pulmonary Drug Delivery Systems's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Pulmonary Drug Delivery Systems
What is the Market Size & CAGR of Pulmonary Drug Delivery Systems market in 2023?
Pulmonary Drug Delivery Systems Industry Analysis
Pulmonary Drug Delivery Systems Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Pulmonary Drug Delivery Systems Market Analysis Report by Region
Europe Pulmonary Drug Delivery Systems Market Report:
The European market is poised to grow from $2.86 billion in 2023 to $6.02 billion by 2033, supported by favorable government initiatives aimed at improving respiratory health and increasing investments in research and development of new therapies.Asia Pacific Pulmonary Drug Delivery Systems Market Report:
In the Asia Pacific, the market size is anticipated to grow from $2.06 billion in 2023 to $4.34 billion by 2033, driven by increased healthcare investments and rising chronic respiratory disease prevalence. Countries like India and China are witnessing substantial growth due to expanding healthcare infrastructure and a large population base.North America Pulmonary Drug Delivery Systems Market Report:
North America, the largest market for Pulmonary Drug Delivery Systems, is projected to grow from $3.27 billion in 2023 to $6.87 billion by 2033. This growth is attributed to advanced healthcare facilities, high prevalence of asthma and COPD, and presence of leading industry players who are innovating continuously.South America Pulmonary Drug Delivery Systems Market Report:
In South America, the market is expected to expand from $0.89 billion in 2023 to $1.87 billion by 2033. The growth is propelled by improving healthcare access and increasing awareness regarding respiratory health management.Middle East & Africa Pulmonary Drug Delivery Systems Market Report:
In the Middle East and Africa, the market is expected to grow from $0.93 billion in 2023 to $1.95 billion by 2033, as healthcare systems improve and awareness for advanced therapeutic solutions rises.Tell us your focus area and get a customized research report.
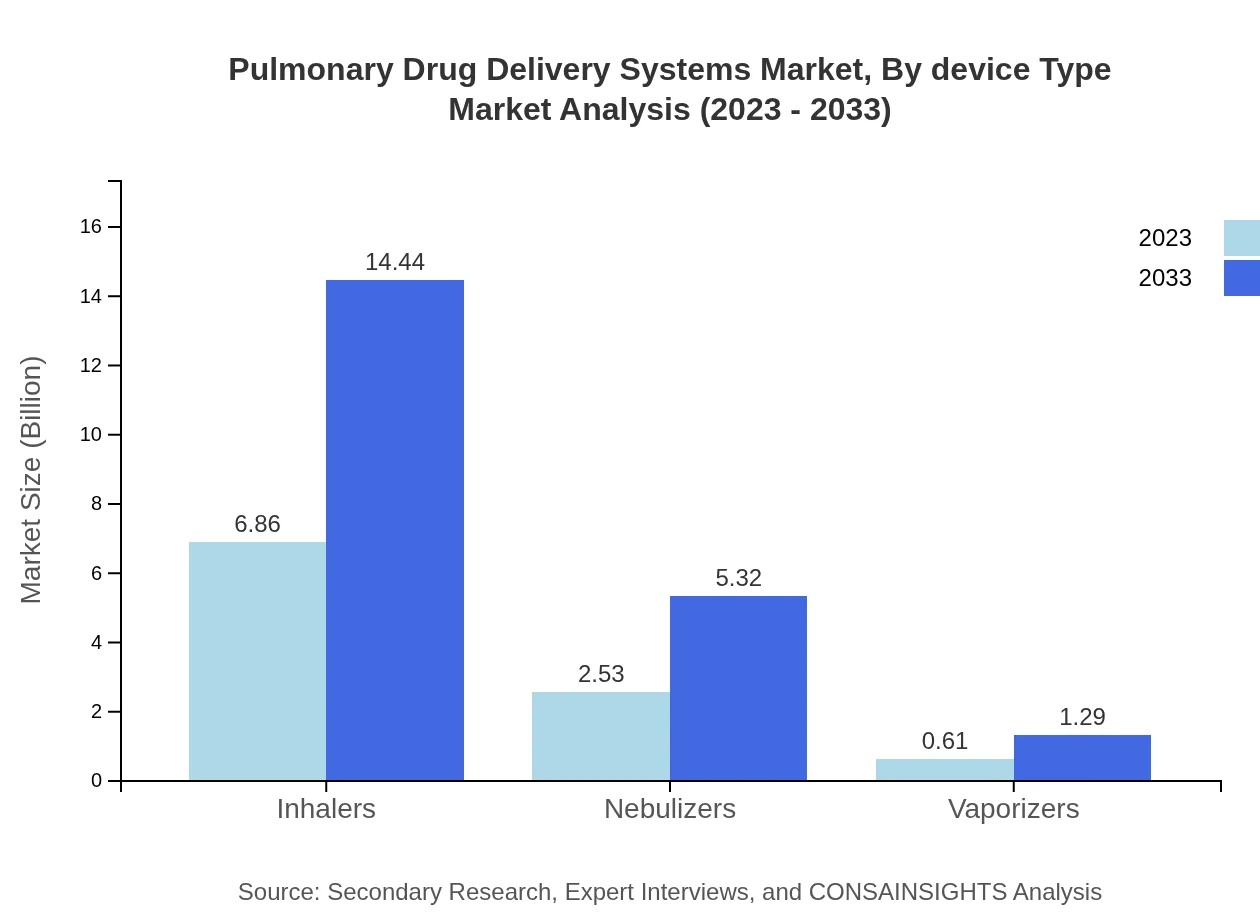
Pulmonary Drug Delivery Systems Market Analysis By Device Type
Inhalers dominate the market with an estimated size of $6.86 billion in 2023, projected to grow to $14.44 billion by 2033, capturing 68.6% of the market share. Nebulizers follow at $2.53 billion, with future growth anticipated to reach $5.32 billion by 2033, making up 25.29%. Vaporizers hold a smaller segment but are also expected to grow from $0.61 billion to $1.29 billion over the same period.
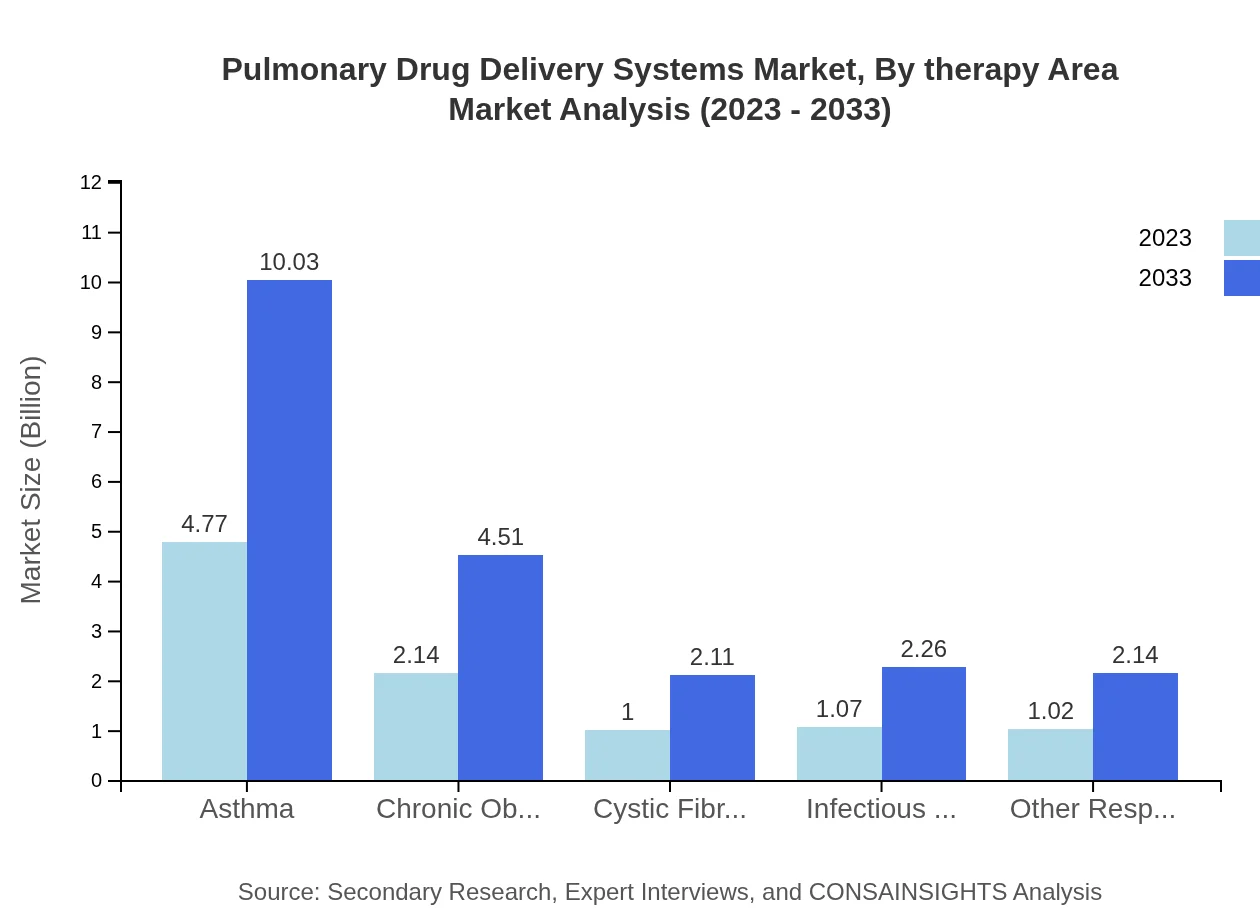
Pulmonary Drug Delivery Systems Market Analysis By Therapy Area
The asthma segment is the largest contributor, valued at $4.77 billion in 2023 and expected to reach $10.03 billion by 2033, representing a 47.66% market share. COPD accounts for $2.14 billion with growth to $4.51 billion, while infectious diseases and other respiratory conditions are also vital segments contributing to overall market growth.
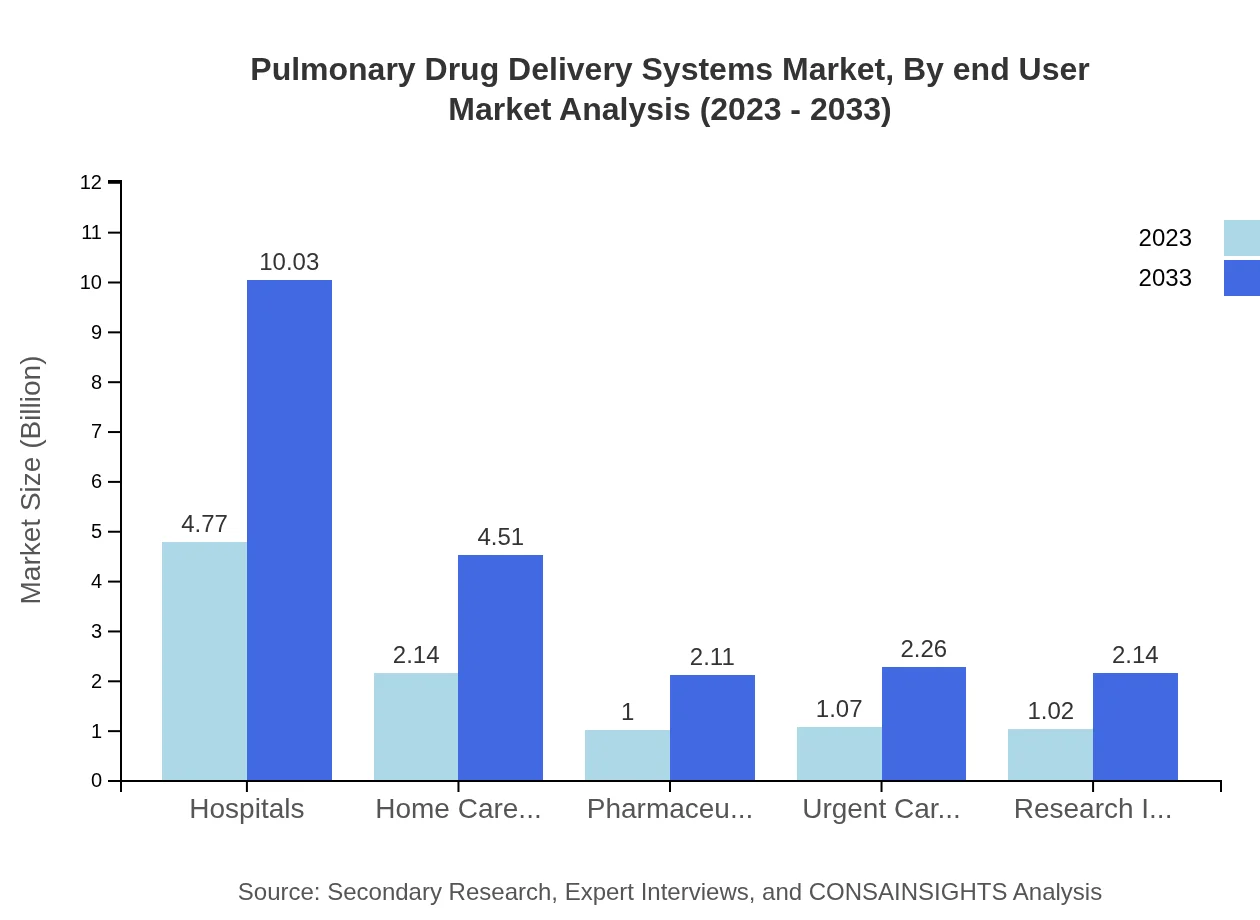
Pulmonary Drug Delivery Systems Market Analysis By End User
Hospitals lead the segment with a market size forecasted to grow from $4.77 billion in 2023 to $10.03 billion by 2033, maintaining a 47.66% share. Home care settings are growing at a similar pace, from $2.14 billion to $4.51 billion, showing a significant trend towards at-home treatment options.
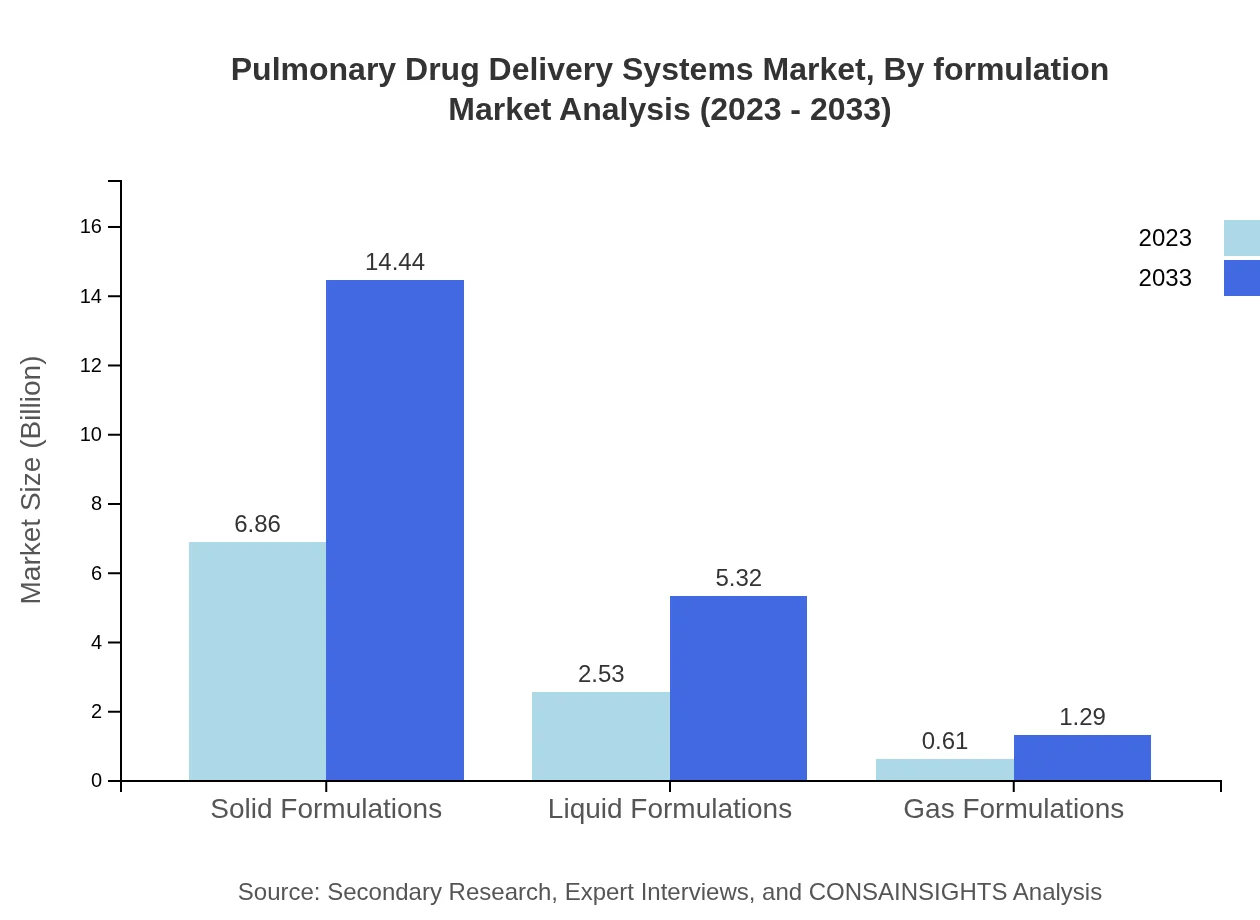
Pulmonary Drug Delivery Systems Market Analysis By Formulation
Solid formulations dominate the market space, expected to grow from $6.86 billion to $14.44 billion, representing 68.6% of the share. Liquid formulations and gas formulations also contribute significantly, albeit at a smaller scale with projections this segment's size reaching $5.32 billion and $1.29 billion, respectively.
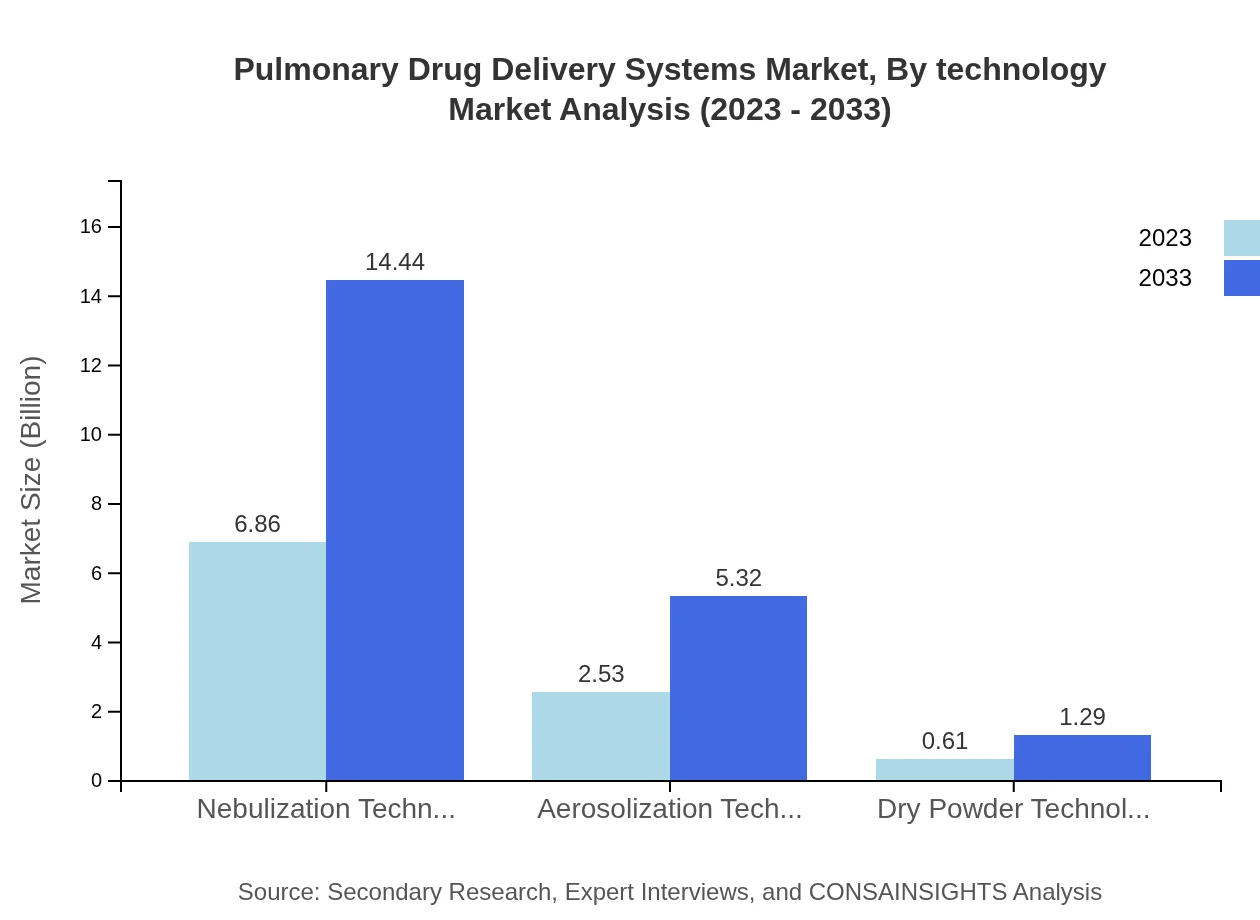
Pulmonary Drug Delivery Systems Market Analysis By Technology
The nebulization technology segment holds the largest share, estimated at $6.86 billion, and expected to grow to $14.44 billion by 2033. Simultaneously, aerosolization and dry powder technologies are experiencing growth, reflecting advancements in pulmonary drug delivery systems making use of varied modes of administration.
Pulmonary Drug Delivery Systems Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Pulmonary Drug Delivery Systems Industry
AstraZeneca:
AstraZeneca is a leading biopharmaceutical company that focuses on the development of treatments for respiratory diseases, contributing significantly to the Pulmonary Drug Delivery Systems market with innovative inhaled therapies.GlaxoSmithKline:
GlaxoSmithKline is renowned for its extensive range of inhalers and aerosol medications, playing a pivotal role in developing advanced therapies for asthma and COPD management.Novartis:
Novartis is a key player in the field focusing on innovative respiratory care solutions, including cutting-edge inhalation technologies that enhance treatment efficacy.Boehringer Ingelheim:
Boehringer Ingelheim specializes in developing inhalable therapies specifically targeting respiratory conditions, contributing significantly to improvements in patient health outcomes.Teva Pharmaceuticals:
Teva Pharmaceuticals is recognized for its commitment to developing generic and innovative pulmonary therapies, thereby expanding access to necessary treatment options.We're grateful to work with incredible clients.









FAQs
What is the market size of pulmonary drug delivery systems?
The global pulmonary drug delivery systems market is valued at approximately $10 billion in 2023 and is projected to grow at a CAGR of 7.5% through 2033. This growth signifies a strong demand and robust innovation in delivery technologies.
What are the key market players or companies in the pulmonary drug delivery systems industry?
The key market players include major pharmaceutical companies specializing in pulmonary therapies, companies designing inhalation devices, and those focusing on drug formulation research. Their innovations significantly shape market trends and drive competition.
What are the primary factors driving the growth in the pulmonary drug delivery systems industry?
Key growth factors include the rising prevalence of respiratory diseases like asthma and COPD, technological advancements in drug delivery systems, and increasing healthcare expenditure. Additionally, the growing preference for home healthcare solutions contributes positively.
Which region is the fastest Growing in the pulmonary drug delivery systems?
The Asia Pacific region is the fastest-growing, with market size expected to increase from $2.06 billion in 2023 to $4.34 billion by 2033. This reveals rapid advancements and investments in healthcare technology within the region.
Does ConsaInsights provide customized market report data for the pulmonary drug delivery systems industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the pulmonary drug delivery systems sector. This can include detailed insights into market trends, growth projections, and competitive analyses.
What deliverables can I expect from this pulmonary drug delivery systems market research project?
Typical deliverables include comprehensive market analysis reports, trends forecasting, segmentation data, insights on regulatory landscape, and competitor analyses. These reports equip stakeholders with detailed information for strategic decision-making.
What are the market trends of pulmonary drug delivery systems?
Current trends include increased adoption of nebulization and aerosolization technologies, rising demand for portable inhalers, and the development of smart inhalers equipped with digital capabilities. These trends reflect a shift towards improved patient experience and adherence.