Pulmonary Function Testing Devices Market Report
Published Date: 31 January 2026 | Report Code: pulmonary-function-testing-devices
Pulmonary Function Testing Devices Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Pulmonary Function Testing Devices market, focusing on market size, growth trends, segmentation, regional insights, and technology advancements from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
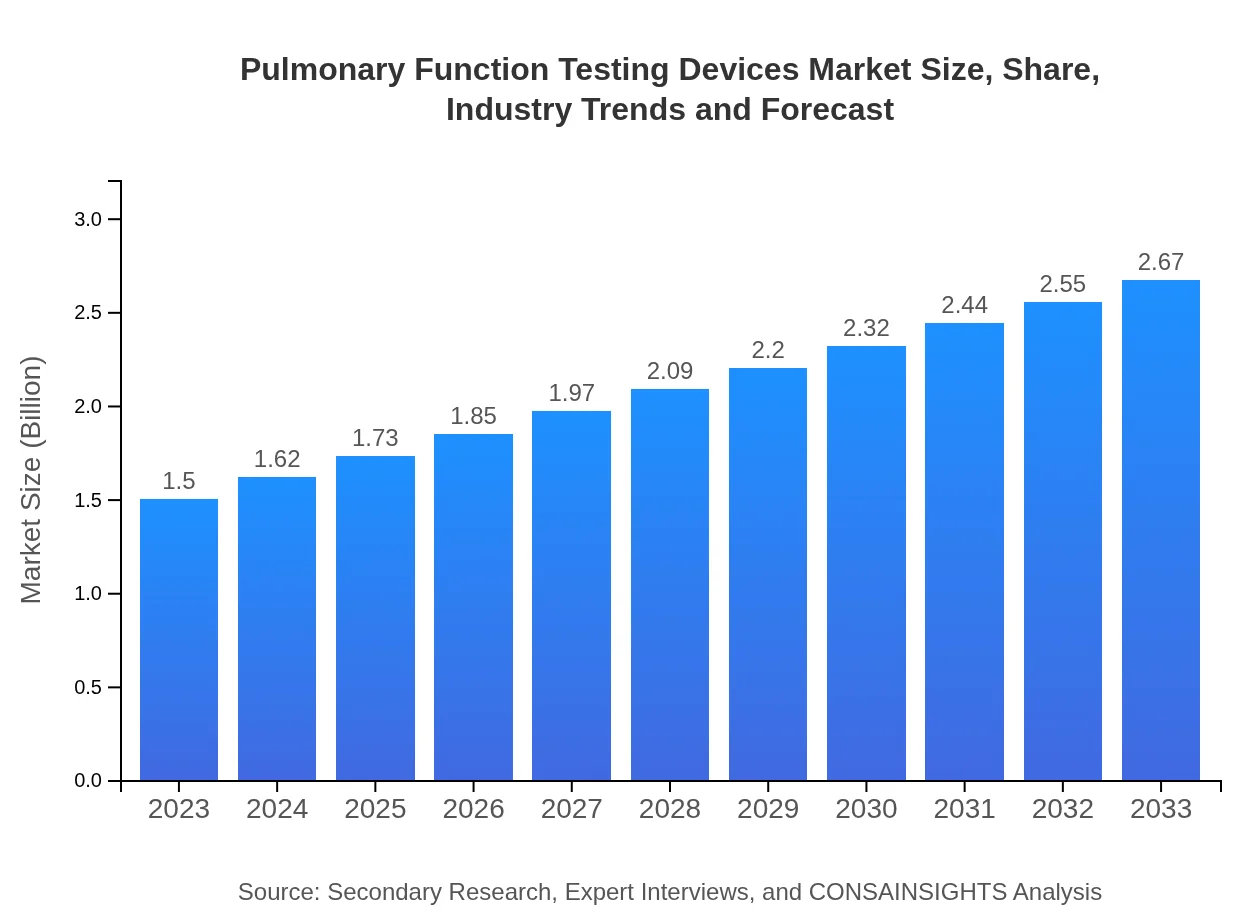
| 2023 Market Size | $1.50 Billion |
| CAGR (2023-2033) | 5.8% |
| 2033 Market Size | $2.67 Billion |
| Top Companies | CareFusion Corporation, Fukuda Sangyo Co., Ltd., MediSoft, Getinge Group, Schiller AG |
| Last Modified Date | 31 January 2026 |
Pulmonary Function Testing Devices Market Overview
Customize Pulmonary Function Testing Devices Market Report market research report
- ✔ Get in-depth analysis of Pulmonary Function Testing Devices market size, growth, and forecasts.
- ✔ Understand Pulmonary Function Testing Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Pulmonary Function Testing Devices
What is the Market Size & CAGR of Pulmonary Function Testing Devices market in 2023?
Pulmonary Function Testing Devices Industry Analysis
Pulmonary Function Testing Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Pulmonary Function Testing Devices Market Analysis Report by Region
Europe Pulmonary Function Testing Devices Market Report:
The European market is expected to grow from $0.42 billion in 2023 to $0.75 billion in 2033, fueled by advancements in medical technology and stringent regulations emphasizing quality healthcare. The United Kingdom and Germany are significant contributors to this market, with ongoing projects aimed at improving respiratory health.Asia Pacific Pulmonary Function Testing Devices Market Report:
In the Asia Pacific region, the market is projected to grow from $0.30 billion in 2023 to $0.54 billion in 2033, driven by increasing urbanization, rising pollution levels, and a growing prevalence of respiratory diseases. Countries like India and China are experiencing a surge in demand for advanced pulmonary testing devices as healthcare infrastructure continues to improve.North America Pulmonary Function Testing Devices Market Report:
North America is currently the largest market with an estimated value of $0.49 billion in 2023, anticipated to reach $0.87 billion by 2033. The robust growth is attributed to high prevalence rates of chronic respiratory diseases, developed healthcare infrastructure, and significant investments in innovative healthcare technologies.South America Pulmonary Function Testing Devices Market Report:
The South American market, although smaller, is expected to grow from $0.08 billion in 2023 to $0.13 billion by 2033. This growth is primarily driven by increasing healthcare investments and rising awareness regarding respiratory illnesses. Brazil and Argentina are key markets in this region, benefiting from enhanced healthcare services.Middle East & Africa Pulmonary Function Testing Devices Market Report:
In the Middle East and Africa, the market is projected to rise from $0.21 billion in 2023 to $0.37 billion by 2033. Increasing healthcare spending and improved access to modern healthcare facilities are key drivers of growth in this region, particularly in the Gulf Cooperation Council (GCC) countries.Tell us your focus area and get a customized research report.
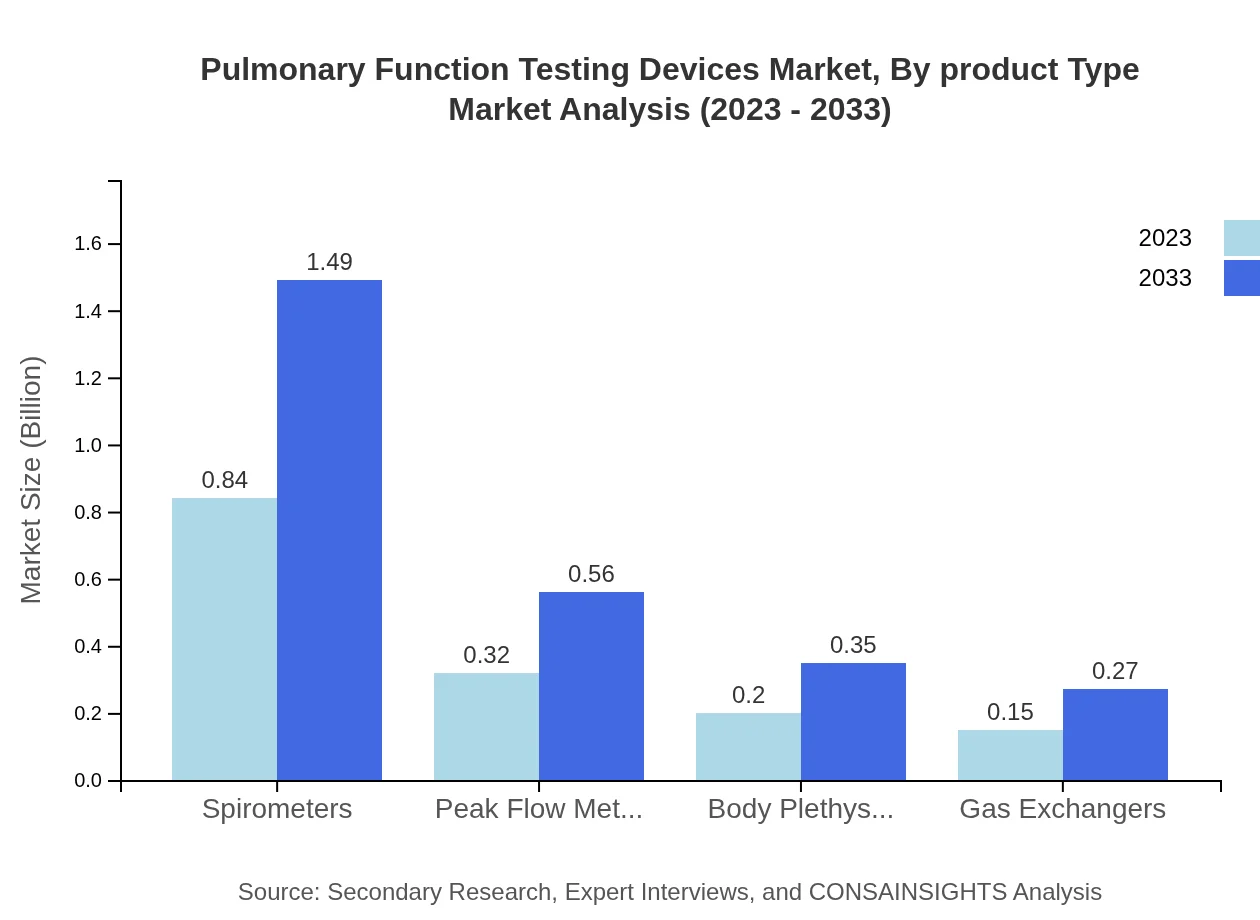
Pulmonary Function Testing Devices Market Analysis By Product Type
The product type segment reveals that spirometers lead the market, with a size of $0.84 billion in 2023, projected to reach $1.49 billion by 2033. Peak flow meters follow at $0.32 billion (2023) to $0.56 billion (2033), while body plethysmographs and gas exchangers represent smaller market shares, yet show significant growth potential in niche applications.
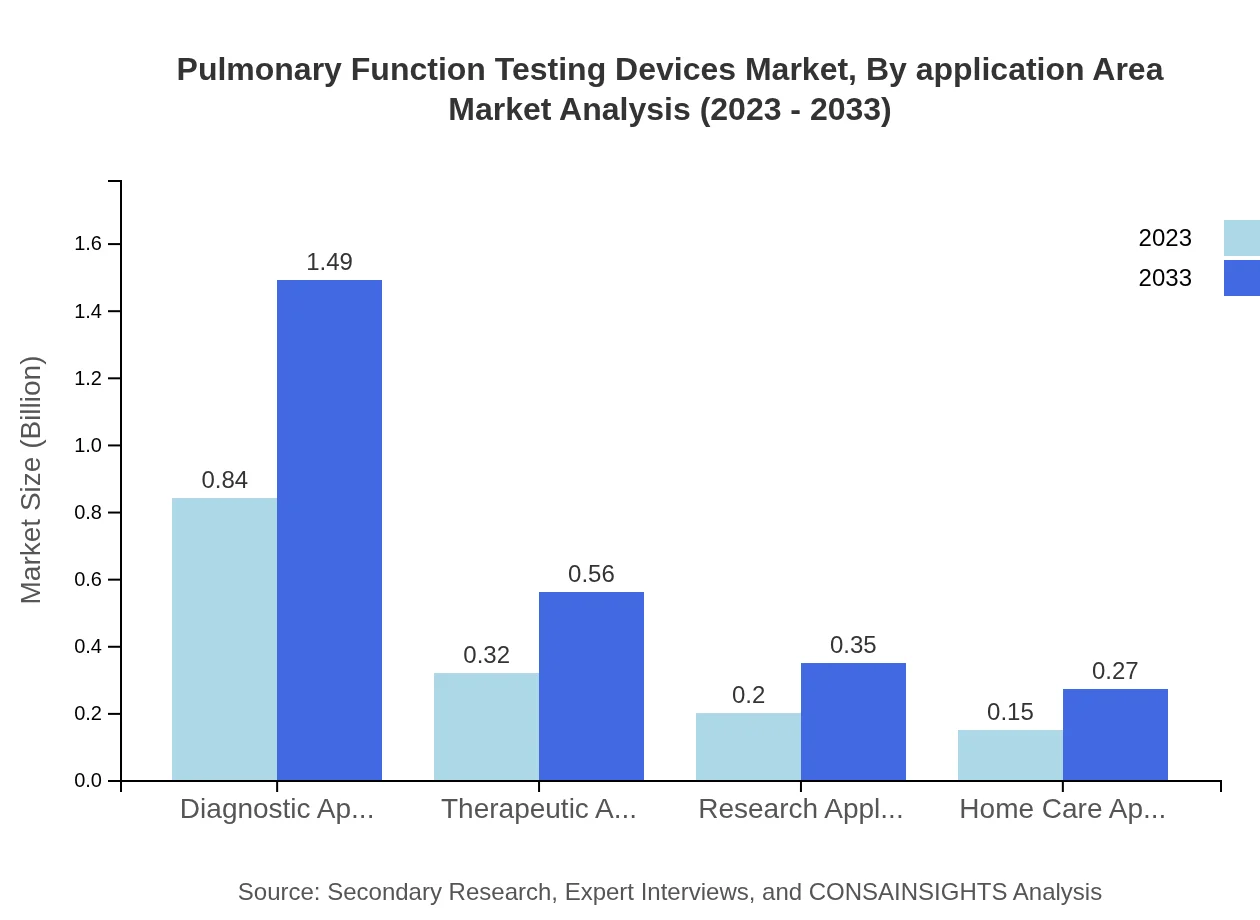
Pulmonary Function Testing Devices Market Analysis By Application Area
In terms of application, diagnostic applications dominate the market, expected to grow from $0.84 billion in 2023 to $1.49 billion by 2033. This is closely followed by therapeutic applications, which will grow to $0.56 billion, highlighting the continuous need for effective treatment approaches in managing respiratory disorders. Research and home care applications also demonstrate promising growth.
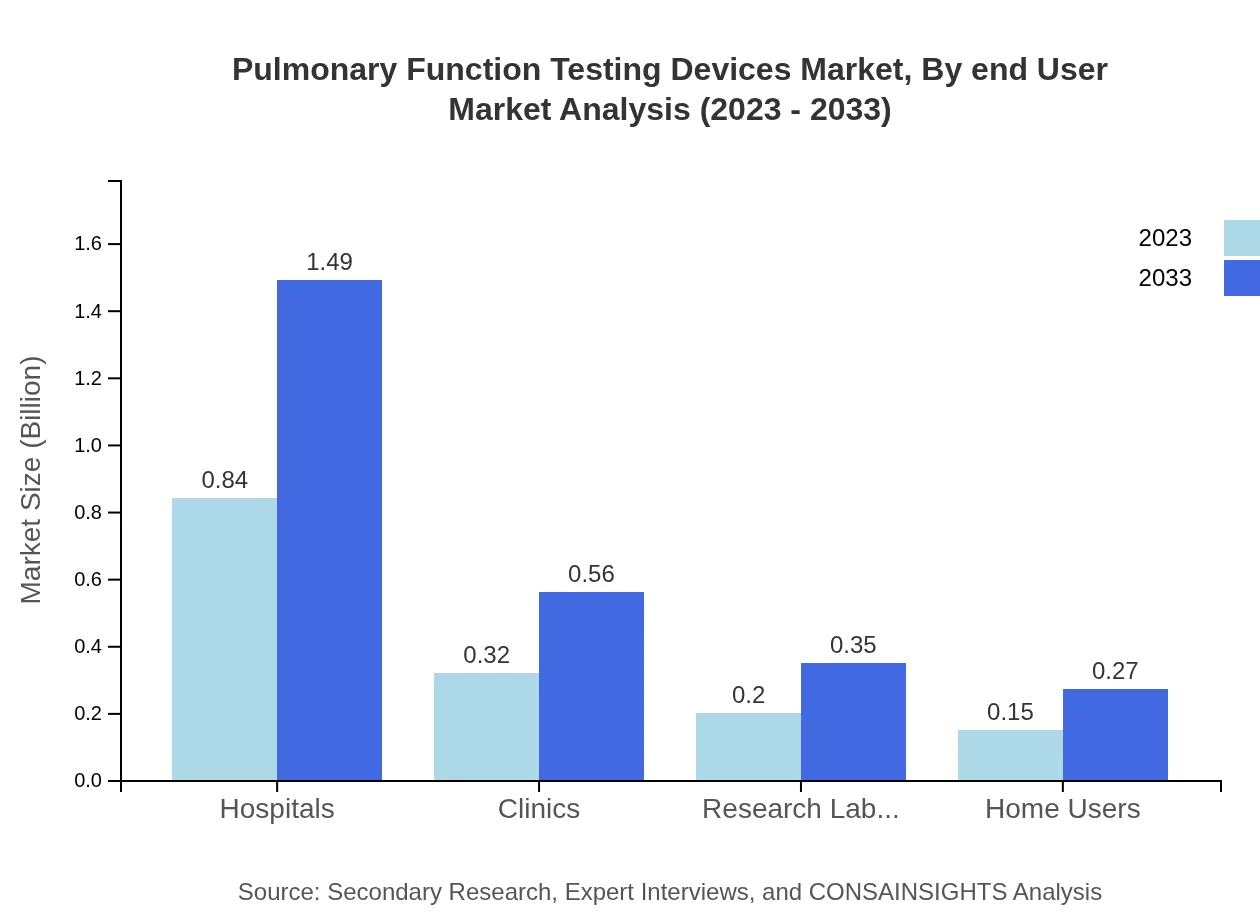
Pulmonary Function Testing Devices Market Analysis By End User
Hospitals account for the largest market share at 55.79% in 2023, with a market size of $0.84 billion, projected to reach $1.49 billion by 2033. Clinics and research laboratories also represent significant shares at 21.05% and 13.04%, respectively, while the home user sector is increasingly capturing interest due to a rising trend in home healthcare.
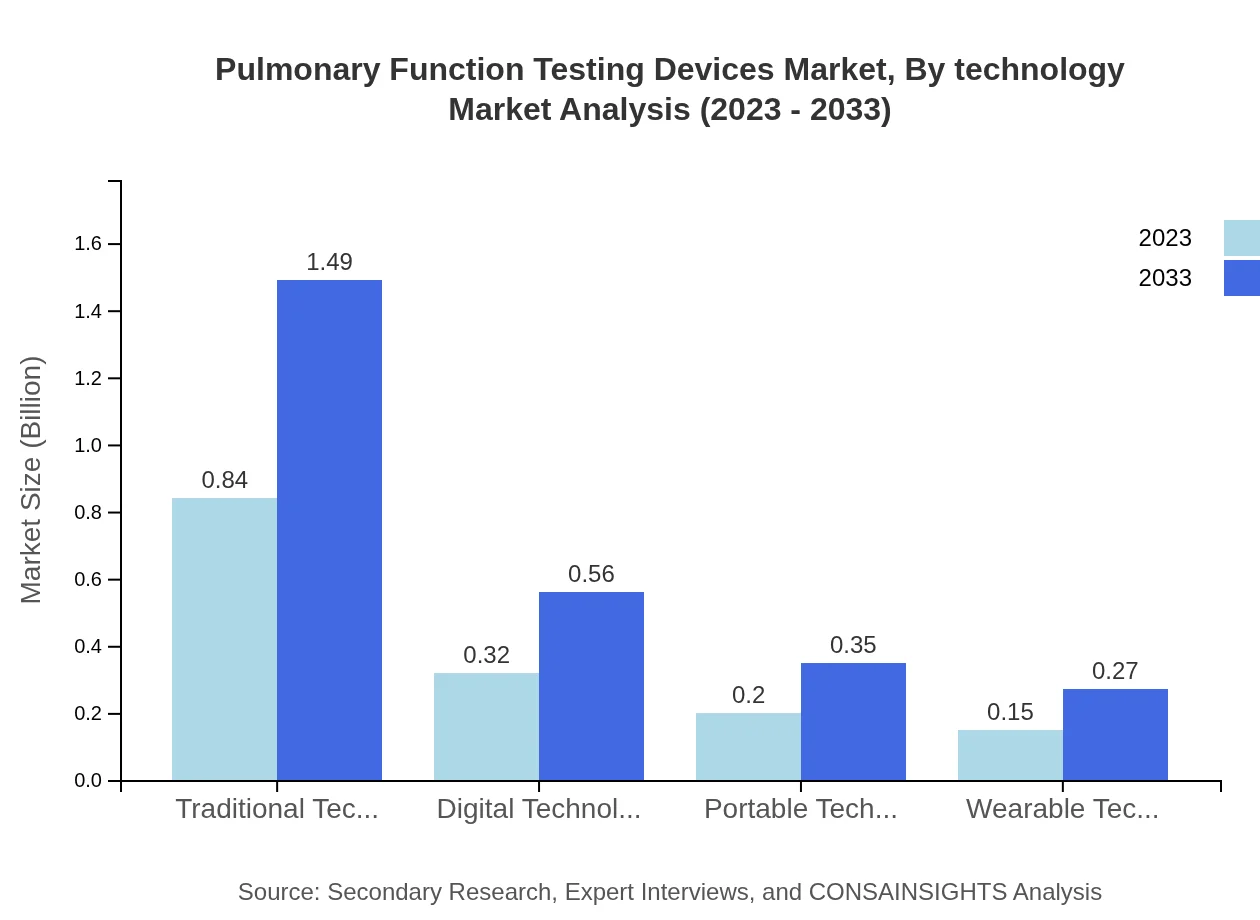
Pulmonary Function Testing Devices Market Analysis By Technology
The technology segment is dominated by traditional technology, with a market size of $0.84 billion in 2023, and is expected to grow to $1.49 billion by 2033. Digital technology holds a significant share, reflecting the industry's shift towards advanced digital diagnostics. Portable and wearable technologies are gaining traction as they allow for greater patient adherence and real-time data collection.
Pulmonary Function Testing Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Pulmonary Function Testing Devices Industry
CareFusion Corporation:
A leading manufacturer of innovative respiratory devices, specializing in critical care and laparoscopic surgery products.Fukuda Sangyo Co., Ltd.:
Recognized for its contributions to pulmonary function testing equipment, focusing on enhancing diagnostic accuracy and patient comfort.MediSoft:
A key player in developing advanced pulmonary function testing solutions, known for its cutting-edge technology and integration capabilities.Getinge Group:
Engaged in advanced medical technology development to improve the quality of care in healthcare.Schiller AG:
A Swiss-based company specializing in medical devices, with a strong portfolio in respiratory diagnostic devices.We're grateful to work with incredible clients.









FAQs
What is the market size of pulmonary Function Testing Devices?
The global market for pulmonary function testing devices is valued at approximately $1.5 billion in 2023, with a projected CAGR of 5.8% from 2023 to 2033.
What are the key market players or companies in the pulmonary Function Testing Devices industry?
Key players in the pulmonary function testing devices market include major companies like Philips Healthcare, Siemens Healthineers, and Respira Labs, all of which contribute to technological advancements and market expansion.
What are the primary factors driving the growth in the pulmonary Function Testing Devices industry?
Growing prevalence of respiratory diseases, advancements in technology, and increasing awareness of early diagnosis are critical drivers behind the robust growth in the pulmonary function testing devices sector.
Which region is the fastest Growing in the pulmonary Function Testing Devices?
The Asia Pacific region is the fastest-growing market for pulmonary function testing devices, with market growth from $0.30 billion in 2023 to an estimated $0.54 billion by 2033, reflecting increasing healthcare investments.
Does ConsaInsights provide customized market report data for the pulmonary Function Testing Devices industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the pulmonary function testing devices industry, enabling clients to obtain detailed insights that align with their market strategies.
What deliverables can I expect from this pulmonary Function Testing Devices market research project?
Deliverables from the pulmonary function testing devices market research project may include comprehensive market analysis reports, detailed segment data, trend forecasts, and strategic recommendations.
What are the market trends of pulmonary Function Testing Devices?
Current trends in the pulmonary function testing devices market include a shift towards digital and portable technologies, increasing adoption of home care applications, and a focus on improving diagnostic capabilities.