Pulmonary Respiratory Drug Delivery Market Report
Published Date: 31 January 2026 | Report Code: pulmonary-respiratory-drug-delivery
Pulmonary Respiratory Drug Delivery Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Pulmonary Respiratory Drug Delivery market, including insights into market dynamics, growth trends, and forecasts from 2023 to 2033. It presents valuable data for stakeholders to understand current conditions and future opportunities.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
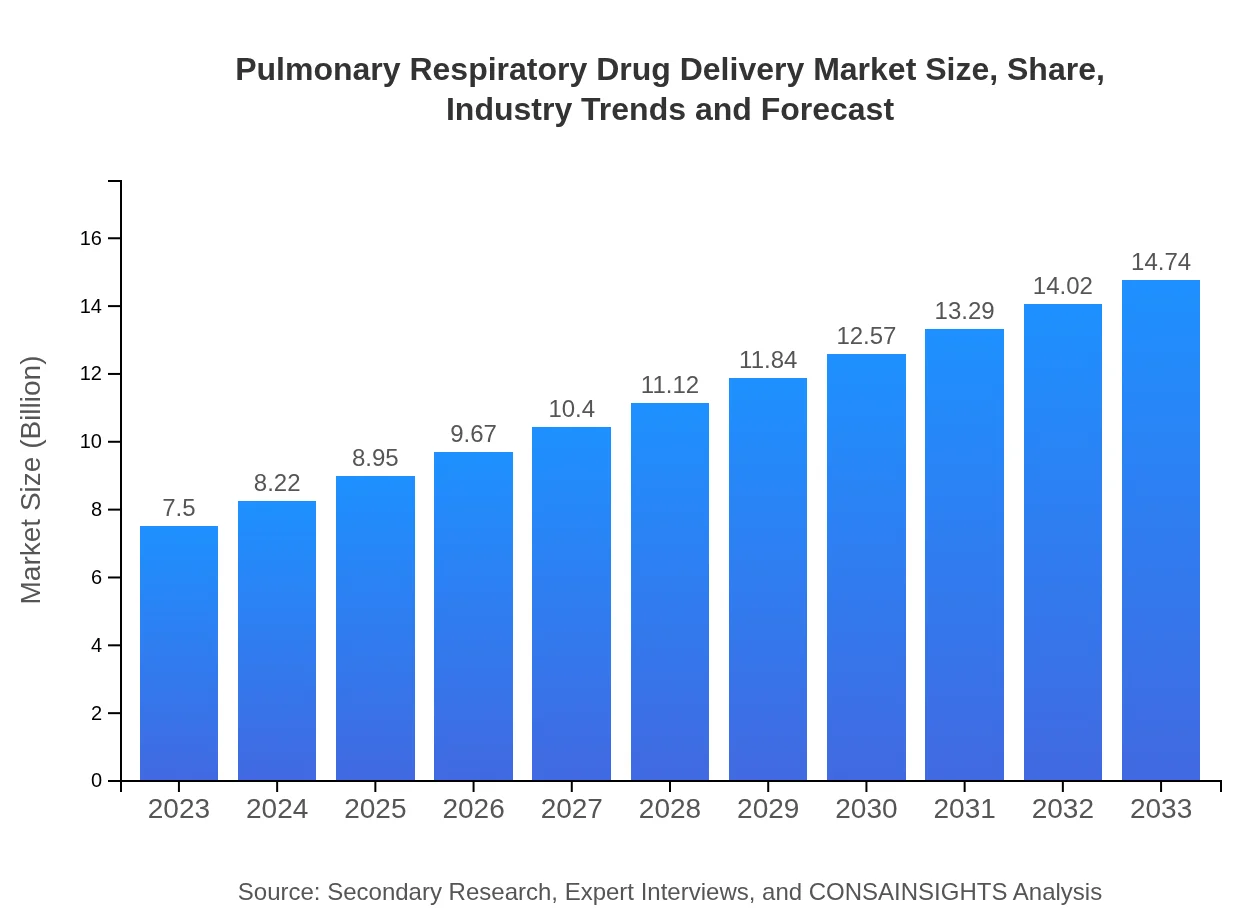
| 2023 Market Size | $7.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $14.74 Billion |
| Top Companies | GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Novartis |
| Last Modified Date | 31 January 2026 |
Pulmonary Respiratory Drug Delivery Market Overview
Customize Pulmonary Respiratory Drug Delivery Market Report market research report
- ✔ Get in-depth analysis of Pulmonary Respiratory Drug Delivery market size, growth, and forecasts.
- ✔ Understand Pulmonary Respiratory Drug Delivery's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Pulmonary Respiratory Drug Delivery
What is the Market Size & CAGR of Pulmonary Respiratory Drug Delivery market in 2023?
Pulmonary Respiratory Drug Delivery Industry Analysis
Pulmonary Respiratory Drug Delivery Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Pulmonary Respiratory Drug Delivery Market Analysis Report by Region
Europe Pulmonary Respiratory Drug Delivery Market Report:
The European market is expected to increase from $2.31 billion in 2023 to $4.54 billion by 2033. This growth trajectory is supported by extensive healthcare infrastructure, rising research funding, and stringent regulatory standards enhancing product efficacy.Asia Pacific Pulmonary Respiratory Drug Delivery Market Report:
In the Asia Pacific region, the market for Pulmonary Respiratory Drug Delivery is projected to grow from $1.42 billion in 2023 to $2.79 billion by 2033, driven by increasing urbanization, pollution levels, and rising awareness of respiratory diseases. Additionally, government initiatives aimed at improving healthcare infrastructure in emerging economies significantly contribute to this growth.North America Pulmonary Respiratory Drug Delivery Market Report:
North America remains the largest market for Pulmonary Respiratory Drug Delivery, anticipated to grow from $2.75 billion in 2023 to $5.41 billion by 2033. Factors include high prevalence rates of asthma and COPD, alongside increasing healthcare expenditure and a focus on innovative treatment methods.South America Pulmonary Respiratory Drug Delivery Market Report:
South America shows a relatively small market, with values expected to rise from $0.13 billion in 2023 to $0.26 billion by 2033. The growth is primarily attributed to potential market expansion as healthcare access improves, though challenges such as economic instability may hinder rapid growth.Middle East & Africa Pulmonary Respiratory Drug Delivery Market Report:
The Middle East and Africa market is projected to grow from $0.89 billion in 2023 to $1.74 billion by 2033. This region's growth can be credited to a rising demand for advanced healthcare solutions and increased investment in the healthcare sector.Tell us your focus area and get a customized research report.
Pulmonary Respiratory Drug Delivery Market Analysis By Product
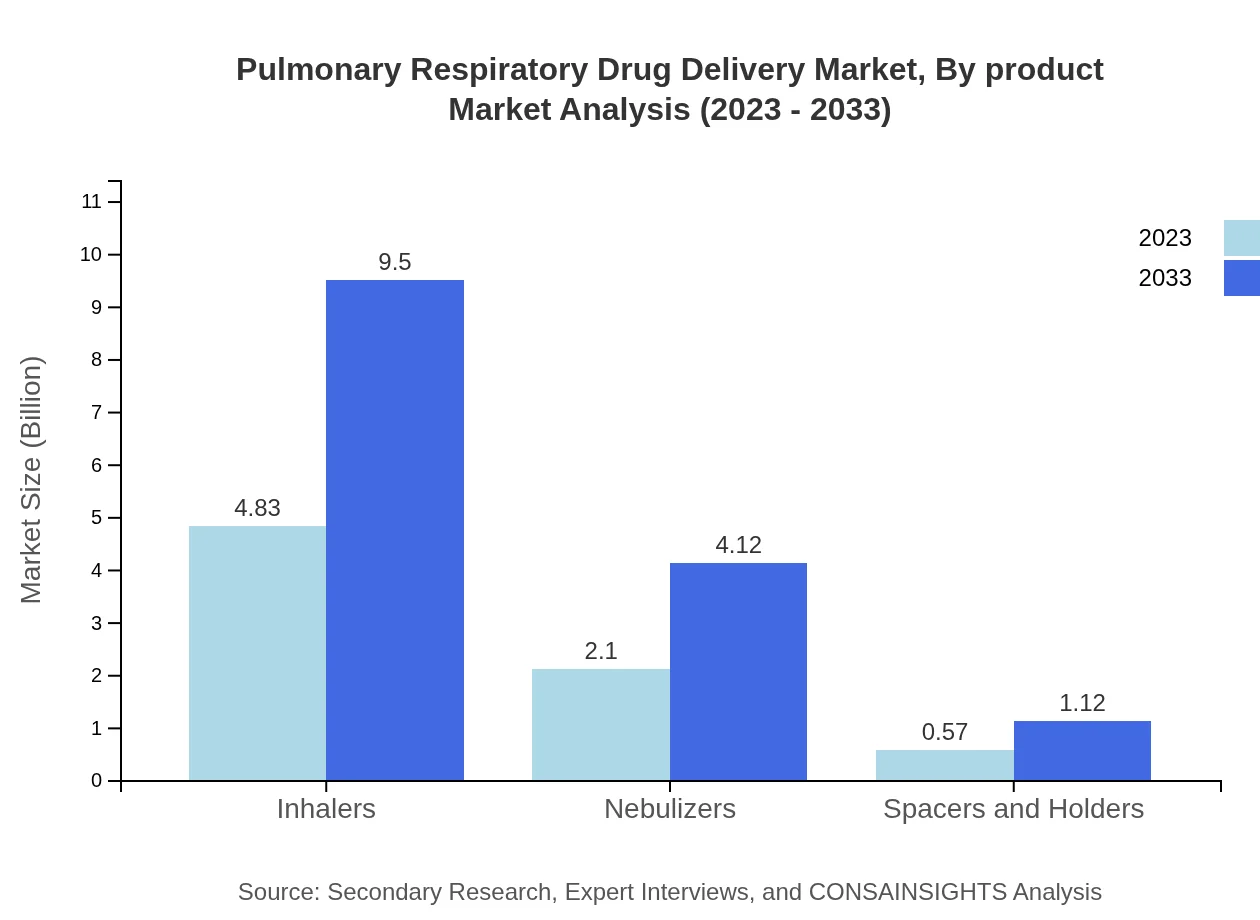
Inhalers are the leading segment, expected to grow from $4.83 billion in 2023 to $9.50 billion by 2033, holding 64.46% market share. Nebulizers follow with a market size of $2.10 billion in 2023, reaching $4.12 billion by 2033, accounting for 27.94% of the market. Spacers and holders contribute to a smaller segment, expanding from $0.57 billion to $1.12 billion, with a 7.6% share.
Pulmonary Respiratory Drug Delivery Market Analysis By Application
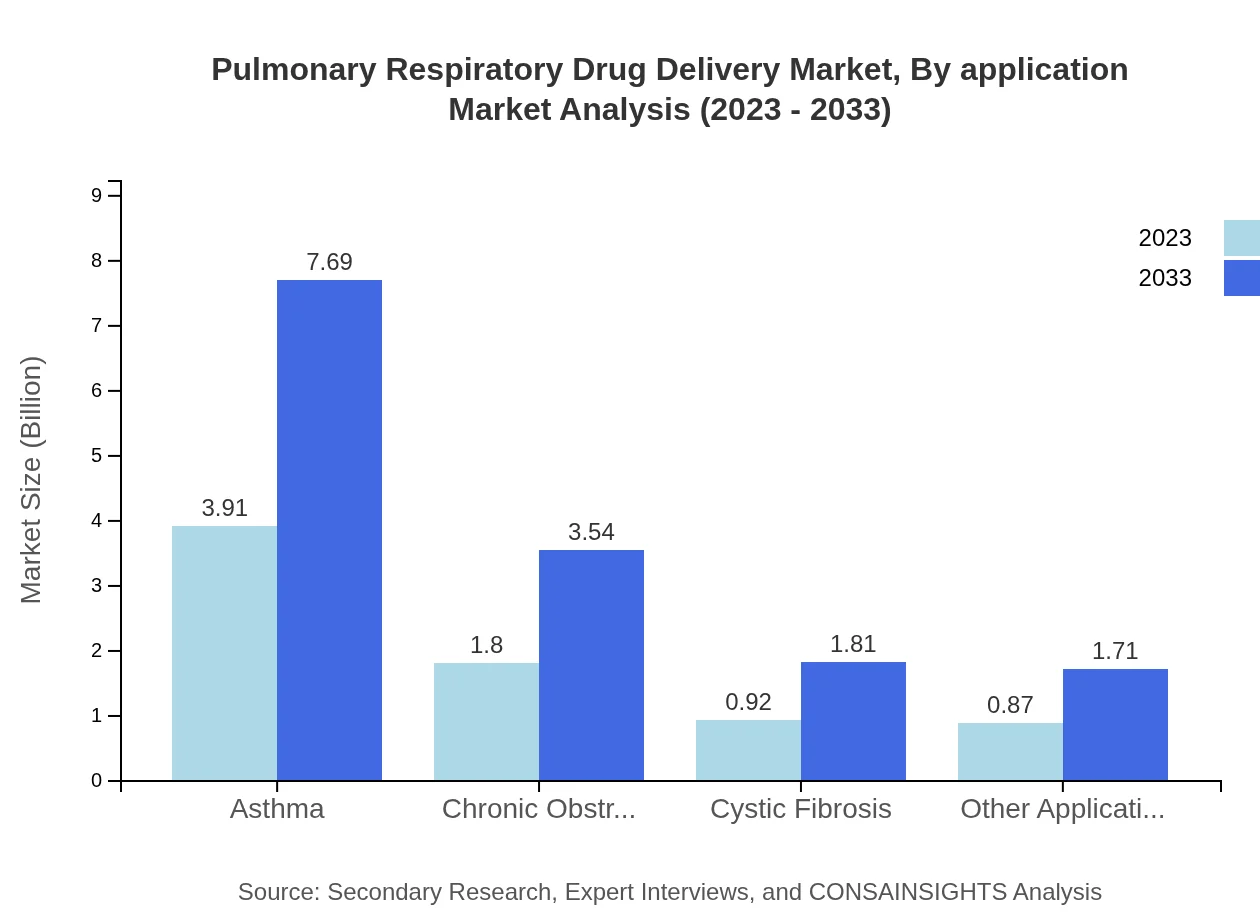
Asthma treatment dominates the application segment, with a market size of $3.91 billion in 2023 and expected to double to $7.69 billion by 2033. COPD accounts for $1.80 billion, potentially growing to $3.54 billion, while cystic fibrosis has a smaller share, moving from $0.92 billion to $1.81 billion. Other application segments comprise $0.87 billion rising to $1.71 billion.
Pulmonary Respiratory Drug Delivery Market Analysis By End User
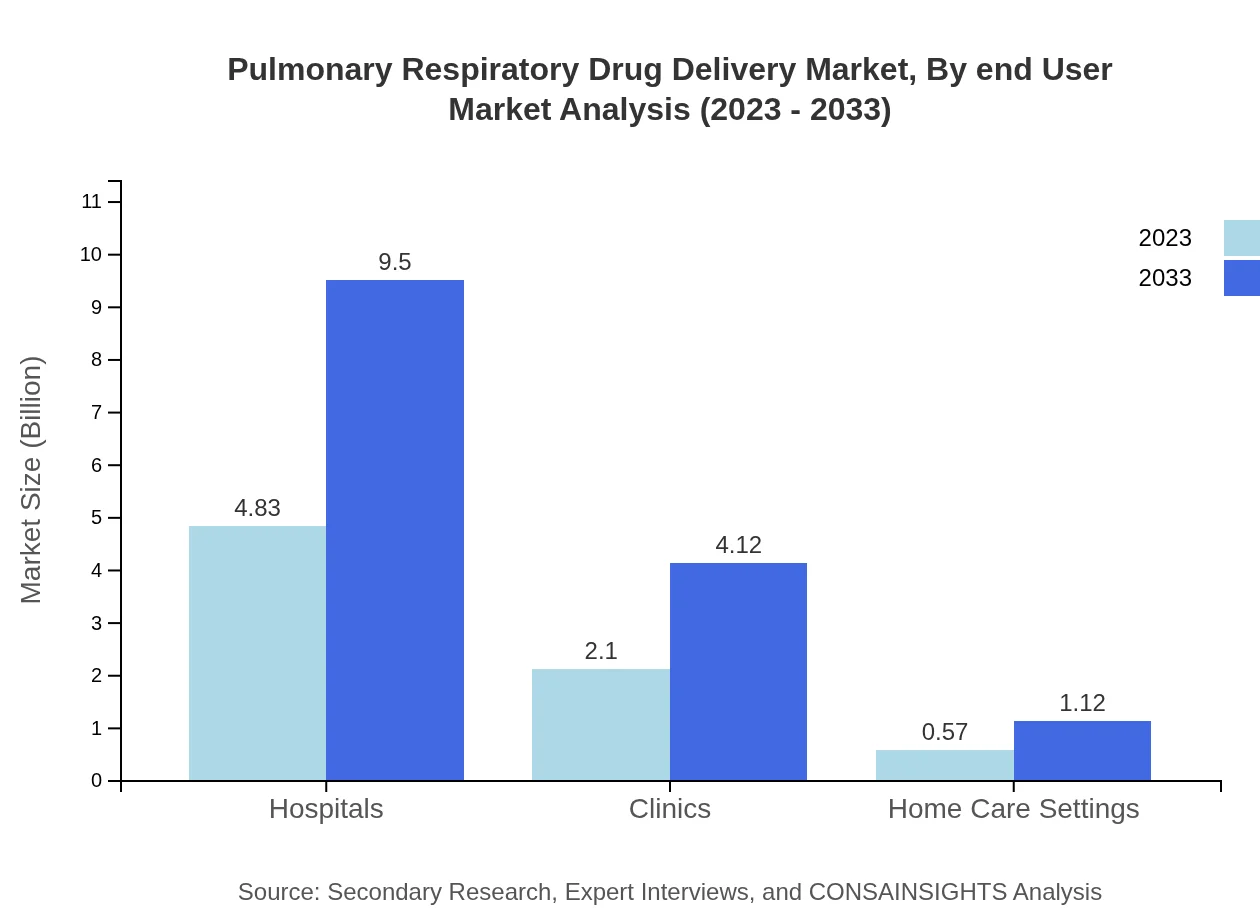
Hospitals remain the predominant end-user segment, valued at $4.83 billion in 2023 and growing to $9.50 billion by 2033, with a consistent share of 64.46%. Clinics are expected to grow from $2.10 billion to $4.12 billion, representing 27.94% of the market. Home care settings, although smaller, are projected to rise from $0.57 billion to $1.12 billion, securing a 7.6% share.
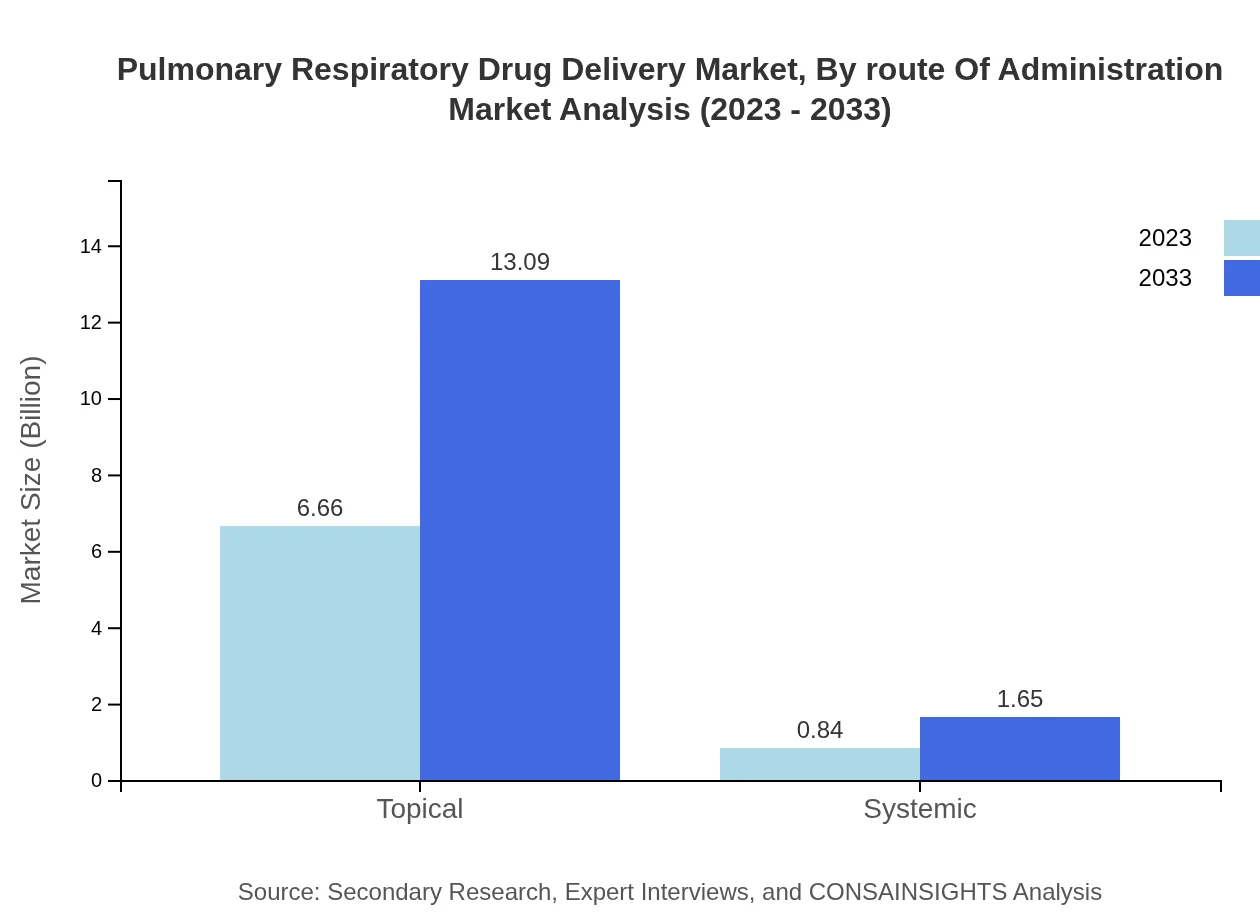
Pulmonary Respiratory Drug Delivery Market Analysis By Route Of Administration
The market is characterized by a substantial emphasis on portable devices, which currently hold an 88.79% share, growing from $6.66 billion in 2023 to $13.09 billion by 2033. Stationary devices, while trailing behind, are expected to grow from $0.84 billion to $1.65 billion, maintaining an 11.21% share.
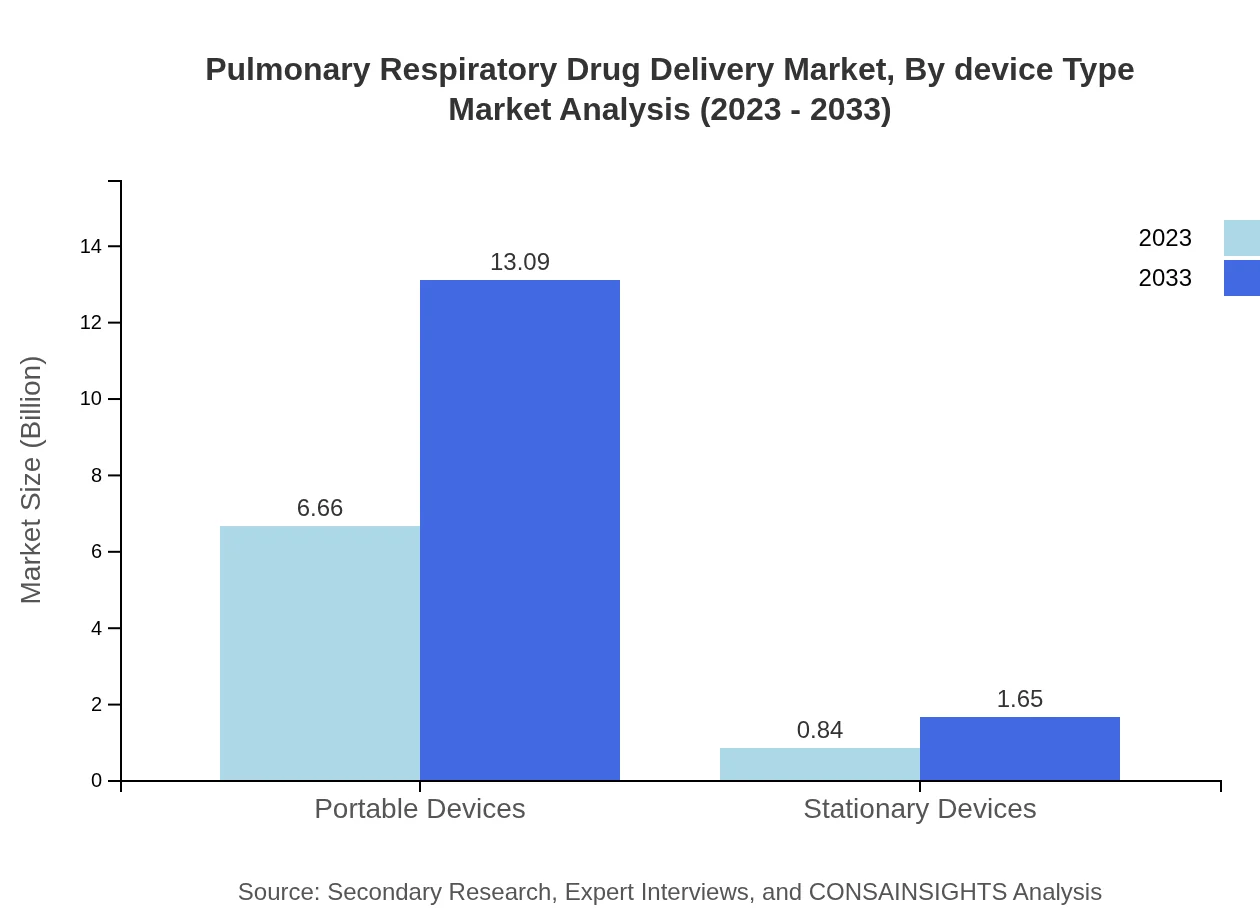
Pulmonary Respiratory Drug Delivery Market Analysis By Device Type
The device segment includes topical delivery systems, which dominate with an 88.79% share, expanding from $6.66 billion to $13.09 billion by 2033, while systemic devices account for 11.21%, showing growth from $0.84 billion to $1.65 billion.
Pulmonary Respiratory Drug Delivery Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Pulmonary Respiratory Drug Delivery Industry
GlaxoSmithKline:
A leading global healthcare company, GlaxoSmithKline specializes in pharmaceuticals that include respiratory therapies, leveraging advanced formulations in the drug delivery space.AstraZeneca:
Known for its significant contributions to the respiratory drug market, AstraZeneca focuses on innovative inhalation therapies, boosting global health through advanced medication solutions.Boehringer Ingelheim:
Boehringer Ingelheim offers a portfolio of products designed for respiratory diseases and engages in research to enhance inhalation technologies.Novartis:
Novartis focuses on strategic R&D to develop novel inhalation therapies, making significant contributions to the Pulmonary Respiratory Drug Delivery market.We're grateful to work with incredible clients.









FAQs
What is the market size of pulmonary Respiratory Drug Delivery?
The pulmonary respiratory drug delivery market is valued at approximately $7.5 billion in 2023, with an anticipated Compound Annual Growth Rate (CAGR) of 6.8% through to 2033. This expansion reflects increasing demand for effective respiratory therapies.
What are the key market players or companies in this pulmonary Respiratory Drug Delivery industry?
Key players in the pulmonary respiratory drug delivery market include major pharmaceutical firms, biotechnology companies, and specialized device manufacturers. Their continuous investment in R&D and strategic partnerships play a critical role in advancing technologies within this industry.
What are the primary factors driving the growth in the pulmonary Respiratory Drug Delivery industry?
The growth in the pulmonary respiratory drug delivery market is driven by rising prevalence of respiratory disorders, advancements in inhalation technology, increasing geriatric population, and a growing emphasis on personalized medicine for better patient outcomes.
Which region is the fastest Growing in the pulmonary Respiratory Drug Delivery?
The Asia Pacific region is the fastest-growing market for pulmonary respiratory drug delivery, projected to increase from $1.42 billion in 2023 to $2.79 billion by 2033, fueled by rising healthcare infrastructure and growing awareness of respiratory diseases.
Does ConsaInsights provide customized market report data for the pulmonary Respiratory Drug Delivery industry?
Yes, ConsaInsights offers customized market report data tailored to the specific needs of clients in the pulmonary respiratory drug delivery industry, ensuring relevant insights and analysis for informed decision-making.
What deliverables can I expect from this pulmonary Respiratory Drug Delivery market research project?
Clients can expect comprehensive deliverables including detailed market analysis, competitive landscape reviews, trend forecasts, regional breakdowns, and actionable insights, all geared towards strategic planning and investment decisions.
What are the market trends of pulmonary Respiratory Drug Delivery?
Current market trends include increasing use of inhalers, a shift towards home care settings for treatment, rising adoption of portable drug delivery devices, and continuous innovation in drug formulations to enhance therapeutic efficacy.