Pyrogen Testing Market Report
Published Date: 31 January 2026 | Report Code: pyrogen-testing
Pyrogen Testing Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the pyrogen testing market from 2023 to 2033, encompassing market size, growth trends, regional insights, and industry analysis to guide stakeholders in strategic decision-making.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
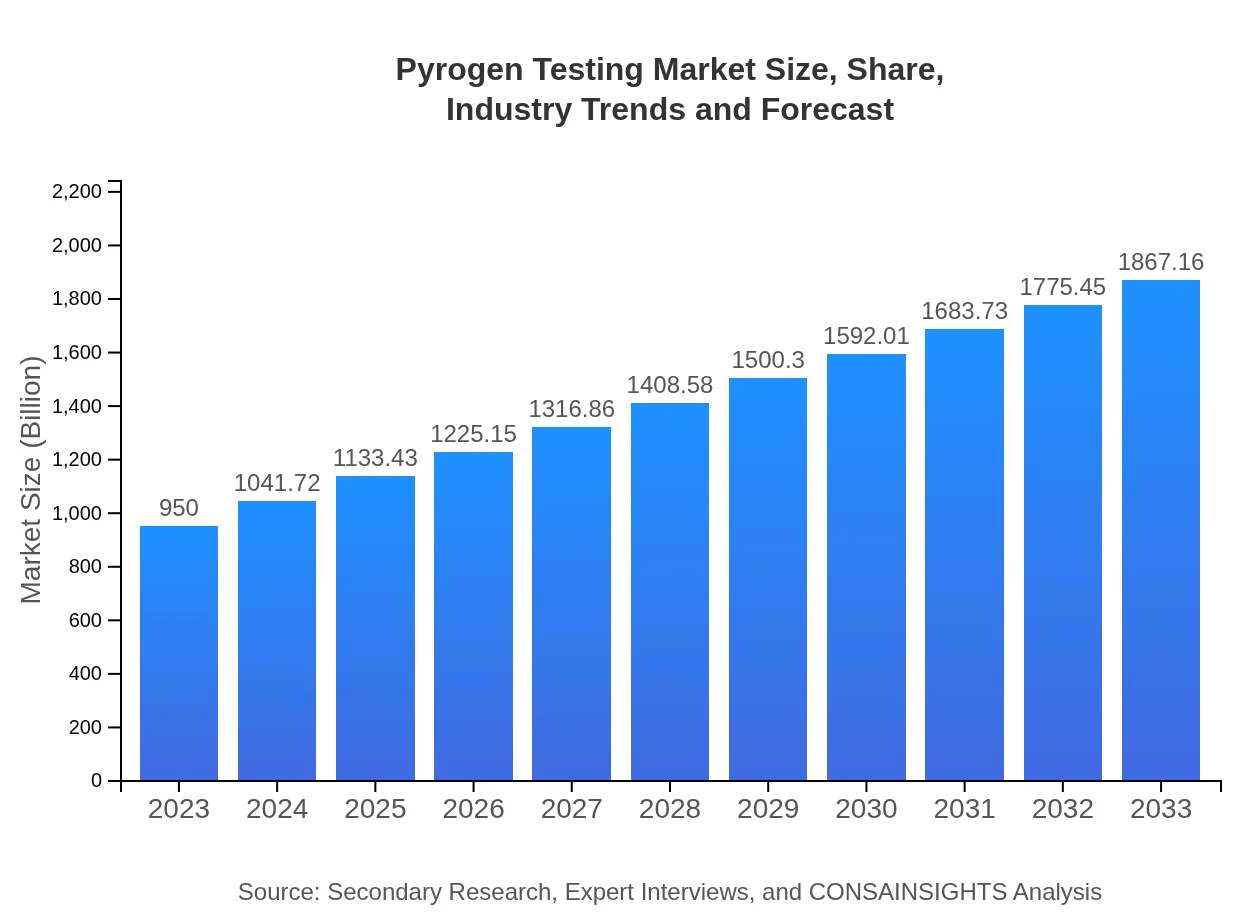
| 2023 Market Size | $950.00 Million |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $1867.16 Million |
| Top Companies | Charles River Laboratories, Thermo Fisher Scientific, Lonza |
| Last Modified Date | 31 January 2026 |
Pyrogen Testing Market Overview
Customize Pyrogen Testing Market Report market research report
- ✔ Get in-depth analysis of Pyrogen Testing market size, growth, and forecasts.
- ✔ Understand Pyrogen Testing's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Pyrogen Testing
What is the Market Size & CAGR of Pyrogen Testing market in 2023?
Pyrogen Testing Industry Analysis
Pyrogen Testing Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Pyrogen Testing Market Analysis Report by Region
Europe Pyrogen Testing Market Report:
The European market is forecasted to grow from 283.67 million USD in 2023 to 557.53 million USD in 2033, influenced by continuous innovations in testing methodologies and stringent safety regulations ramping up demand for reliable testing solutions.Asia Pacific Pyrogen Testing Market Report:
The Asia Pacific region is expected to witness substantial growth, with market size projected to reach 326.19 million USD by 2033, up from 165.97 million in 2023. The expansion is driven by the growth of pharmaceutical industries and increasing regulatory requirements regarding product safety.North America Pyrogen Testing Market Report:
North America remains the largest market for pyrogen testing, with an expected market size of 726.14 million USD in 2033 compared to 369.46 million USD in 2023. Factors include strong regulatory frameworks, high healthcare standards, and advanced testing technologies.South America Pyrogen Testing Market Report:
In South America, the pyrogen testing market is predicted to grow from 44.84 million USD in 2023 to 88.13 million USD by 2033. This growth can be attributed to a rise in local pharmaceutical manufacturing capabilities and improved awareness of testing protocols.Middle East & Africa Pyrogen Testing Market Report:
The Middle East and Africa segment is estimated to reach 169.16 million USD by 2033, up from 86.07 million USD in 2023. Growth is driven by increased pharmaceutical investments and the establishment of quality control labs in the region.Tell us your focus area and get a customized research report.
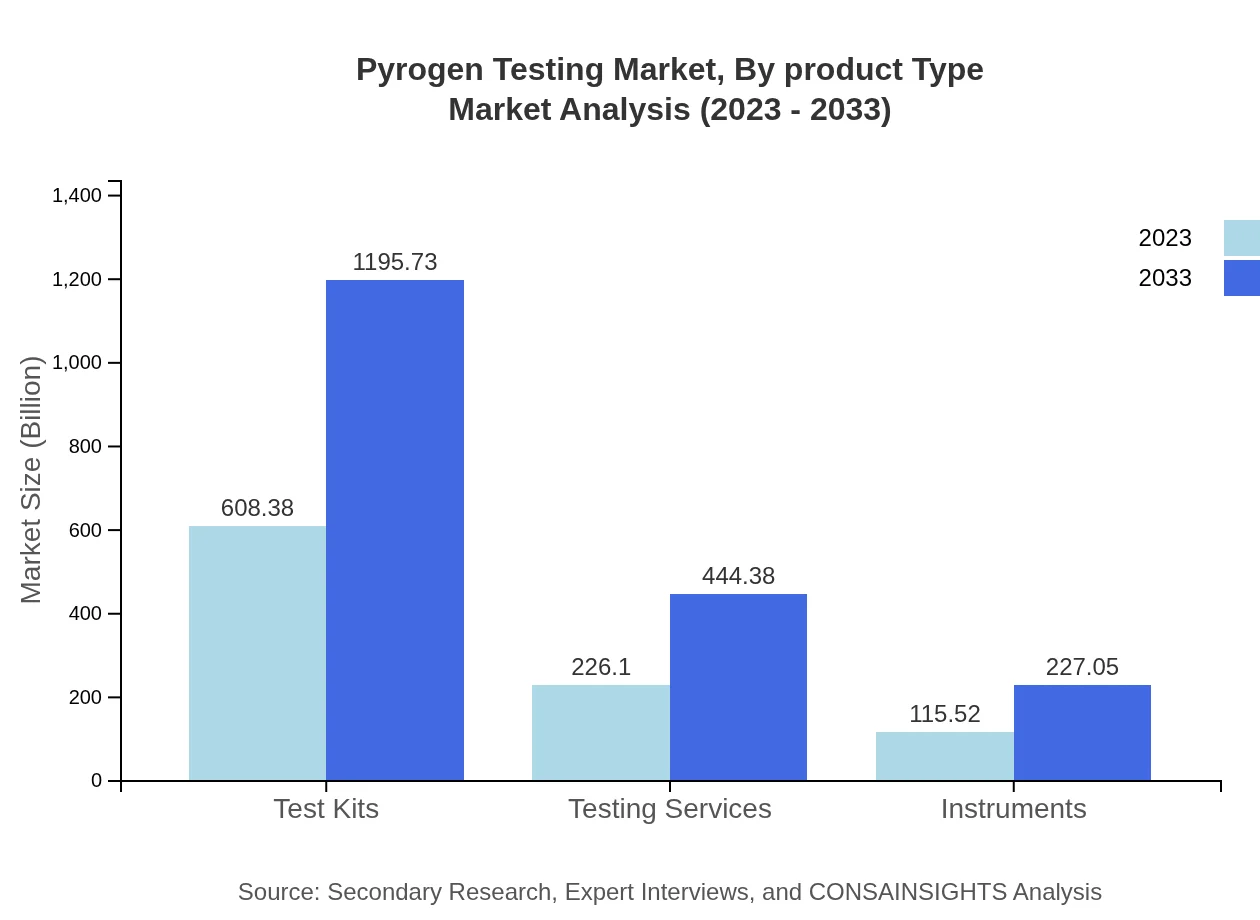
Pyrogen Testing Market Analysis By Product Type
Market segmentation by product type reveals that testing laboratories constitute a significant portion, with estimates of 494.19 million USD in 2023 growing to 971.30 million USD by 2033 (52% market share). Pharmaceutical manufacturers are projected to have a market size increase from 236.84 million USD to 465.48 million USD (25% share), while biotechnology companies will grow from 101.08 million USD to 198.67 million USD (10% share), reflecting slight increases in their market roles.
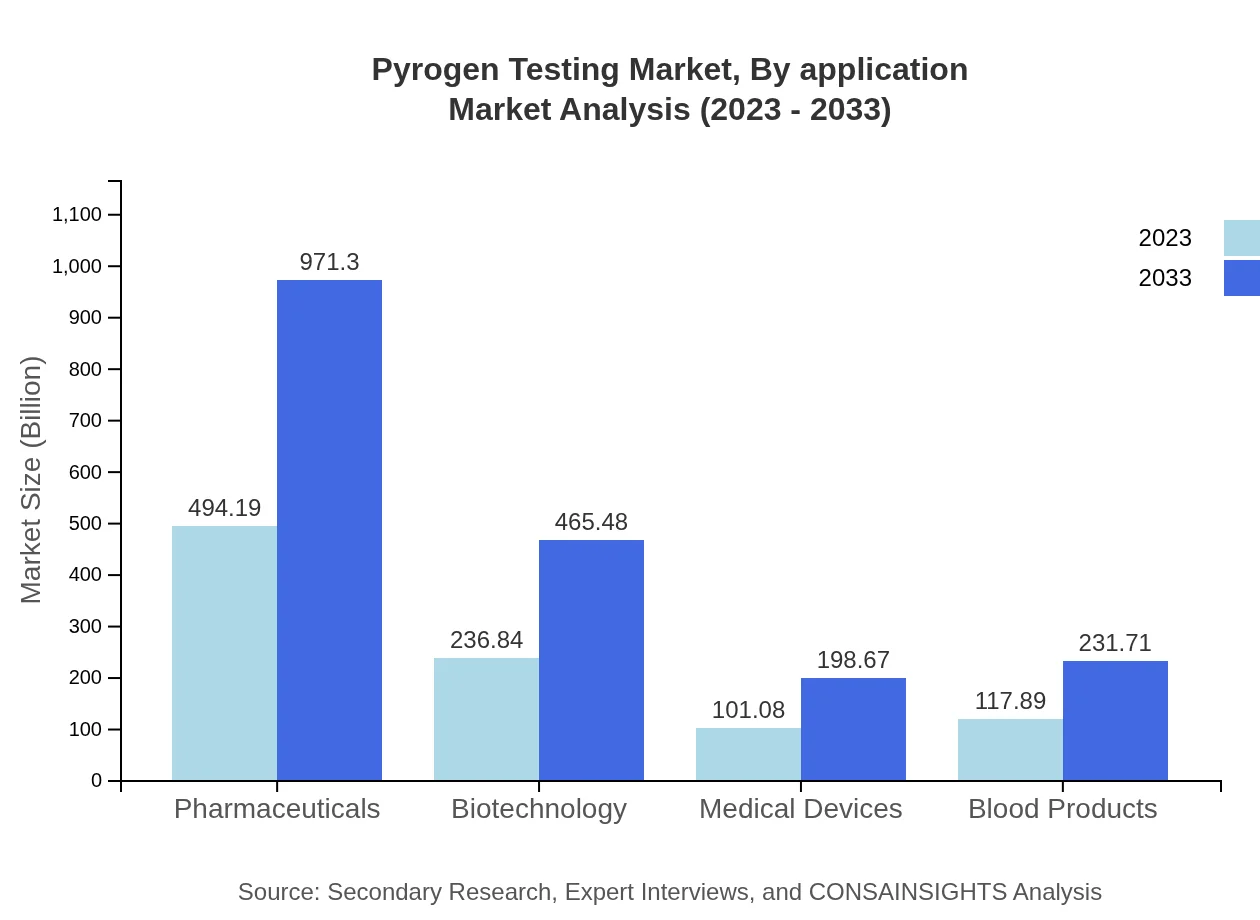
Pyrogen Testing Market Analysis By Application
Different applications show varying performances, with pharmaceuticals leading the segment anticipated to rise from 494.19 million USD to 971.30 million USD (52% share), while biotechnology applications increase in relevance from 236.84 million USD to 465.48 million USD (25% share), and medical devices hold at 117.89 million USD, reaching 231.71 million USD (12% share), marking them as vital components in the pyrogen testing ecosystem.
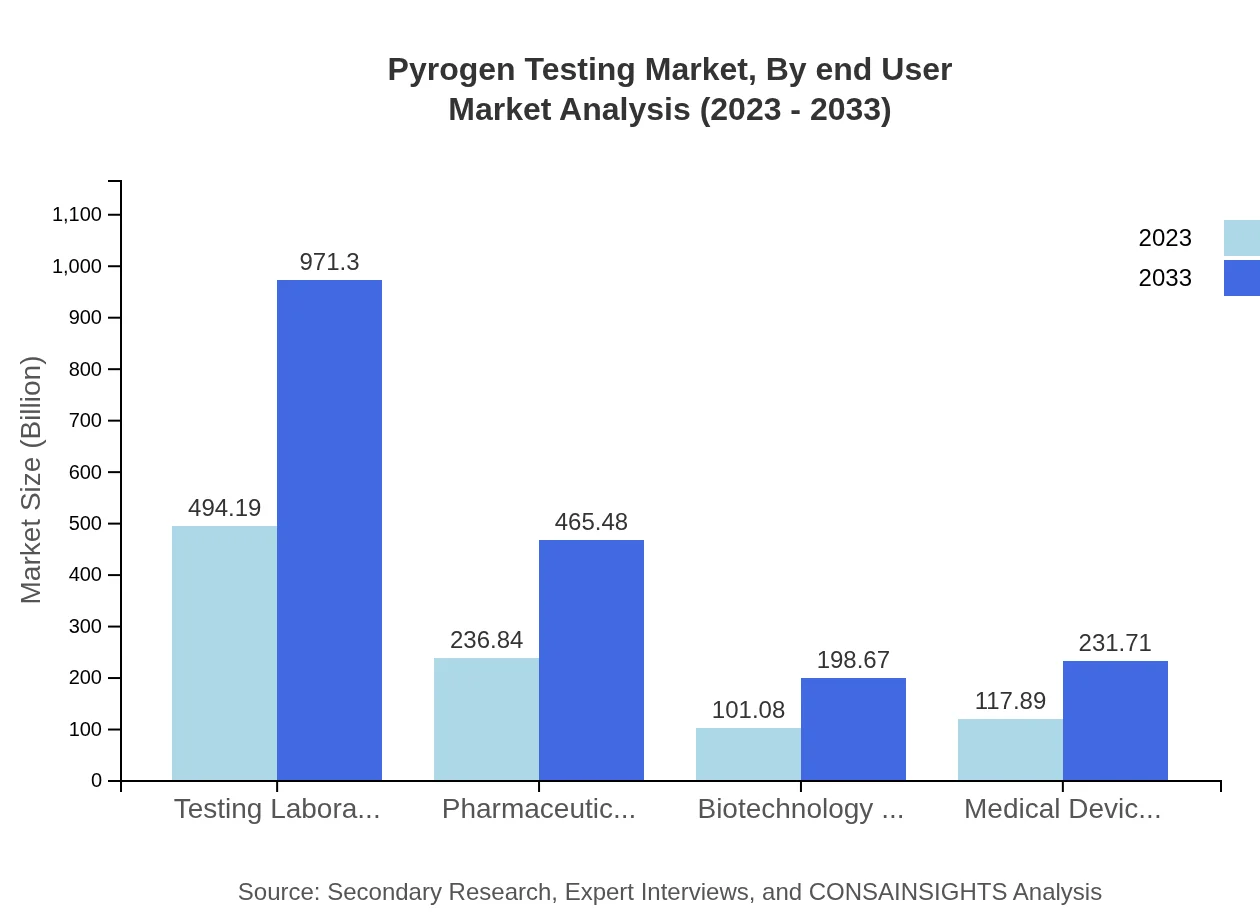
Pyrogen Testing Market Analysis By End User
End-user analysis in the pyrogen testing market indicates that testing laboratories and pharmaceutical manufacturers dominate the landscape – testing laboratories comprise a significant size of 494.19 million USD (52% share) in 2023, poised to double by 2033. Meanwhile, biotechnology companies and medical devices use in testing show rising trajectories from 101.08 million USD to 198.67 million USD, impacting overall testing methodologies.
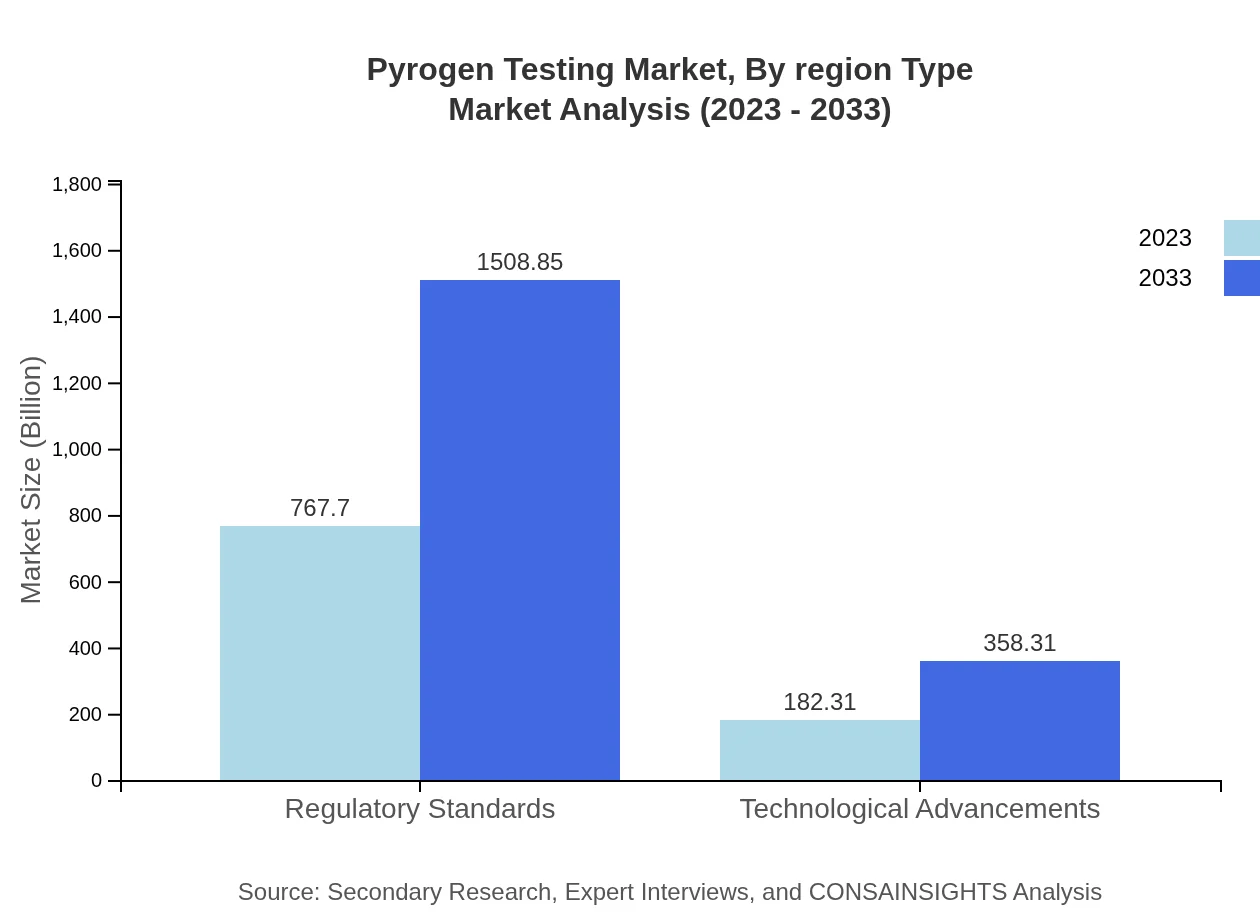
Pyrogen Testing Market Analysis By Region Type
Regional insights convey that North America leads the market due to its structured regulatory environment, followed closely by Europe where market penetration continues to rise. In juxtaposition, emerging markets in Asia Pacific are anticipated to witness rapid growth as manufacturers seek to meet ever-evolving regulatory compliance and consumer safety expectations.
Pyrogen Testing Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Pyrogen Testing Industry
Charles River Laboratories:
A leading provider of preclinical and clinical laboratory services that help pharmaceutical and biotechnology companies develop safe and effective treatments. They offer comprehensive pyrogen testing services.Thermo Fisher Scientific:
This company offers a broad portfolio of pyrogen testing products and technologies aimed at ensuring the safety of biopharmaceuticals. Their innovations play a significant role in market growth.Lonza:
Lonza is a key player in the biotech and pharma sector, providing critical testing solutions adhering to stringent quality standards within the pyrogen testing domain.We're grateful to work with incredible clients.









FAQs
What is the market size of pyrogen Testing?
The global pyrogen testing market is valued at approximately $950 million in 2023, and it is expected to grow at a CAGR of 6.8%, reaching significant expansion by 2033.
What are the key market players or companies in this pyrogen Testing industry?
Key players in the pyrogen testing industry include Charles River Laboratories, Wuxi Apptec, Thermo Fisher Scientific, and Lonza. They dominate through extensive portfolios and significant market share.
What are the primary factors driving the growth in the pyrogen Testing industry?
Growth factors include the increasing demand for biopharmaceuticals, stringent regulatory compliances, technological advancements in testing methods, and rising awareness of product safety among manufacturers.
Which region is the fastest Growing in the pyrogen Testing?
The North American region is the fastest-growing in the pyrogen testing market, projected to grow from $369.46 million in 2023 to $726.14 million by 2033, showcasing significant market potential.
Does ConsaInsights provide customized market report data for the pyrogen Testing industry?
Yes, ConsaInsights offers customized market report data tailored to your specific needs in the pyrogen testing industry, enabling you to gain accurate and relevant insights.
What deliverables can I expect from this pyrogen Testing market research project?
You can expect comprehensive reports including market size analysis, segment data, growth forecasts, competitive landscape insights, trends, and customized business recommendations based on your needs.
What are the market trends of pyrogen Testing?
Current trends in the pyrogen testing market include advancements in test kits, increased adoption of regulatory standards, and rising investments in biotechnology and pharmaceutical sectors.