Radioactive Stents Market Report
Published Date: 31 January 2026 | Report Code: radioactive-stents
Radioactive Stents Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Radioactive Stents market, focusing on key insights, market dynamics, and forecasts for the period 2023 to 2033. It covers market size, segmentation, regional analysis, trends, and competitive landscape in detail.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
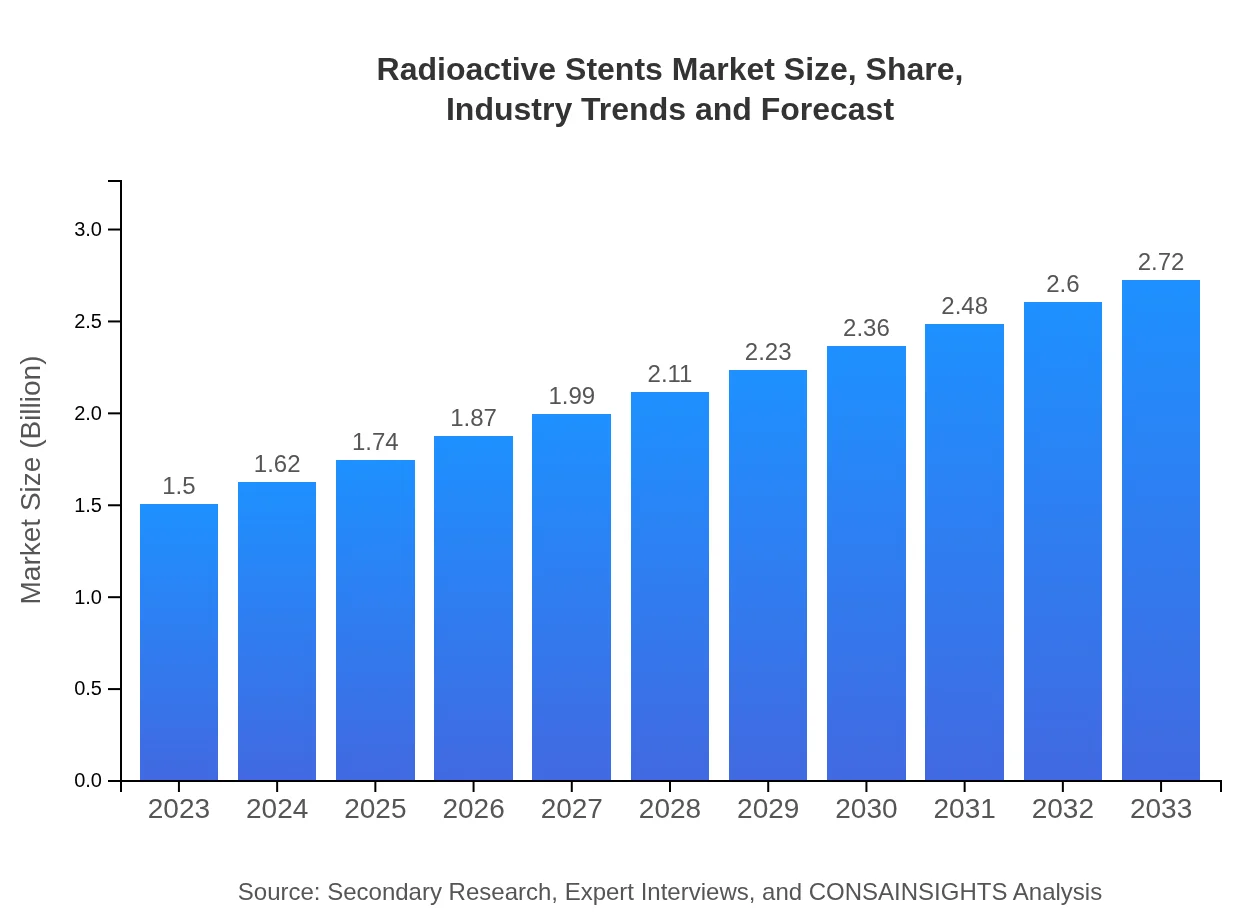
| 2023 Market Size | $1.50 Billion |
| CAGR (2023-2033) | 6.0% |
| 2033 Market Size | $2.72 Billion |
| Top Companies | Boston Scientific Corporation, Abbott Laboratories, Medtronic , Bard Peripheral Vascular, Stryker Corporation |
| Last Modified Date | 31 January 2026 |
Radioactive Stents Market Overview
Customize Radioactive Stents Market Report market research report
- ✔ Get in-depth analysis of Radioactive Stents market size, growth, and forecasts.
- ✔ Understand Radioactive Stents's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Radioactive Stents
What is the Market Size & CAGR of the Radioactive Stents market in 2023?
Radioactive Stents Industry Analysis
Radioactive Stents Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Radioactive Stents Market Analysis Report by Region
Europe Radioactive Stents Market Report:
The European Radioactive Stents market is anticipated to expand from USD 0.44 billion in 2023 to USD 0.80 billion by 2033. Factors contributing to this growth include the rising geriatric population, advancements in medical technologies, and greater access to specialized healthcare services across various European countries.Asia Pacific Radioactive Stents Market Report:
In the Asia Pacific region, the Radioactive Stents market is projected to grow from USD 0.29 billion in 2023 to USD 0.52 billion by 2033. An increasing preference for minimally invasive surgeries and rising healthcare expenditure are key growth drivers in this region, along with the expanding patient population suffering from cardiovascular diseases.North America Radioactive Stents Market Report:
North America dominates the Radioactive Stents market, forecasted to see growth from USD 0.55 billion in 2023 to USD 0.99 billion by 2033. The region benefits from a robust healthcare infrastructure, high patient awareness, and continuous innovation in stent technology. Moreover, the increased prevalence of coronary artery diseases significantly boosts market demand.South America Radioactive Stents Market Report:
South America is expected to see the Radioactive Stents market grow from USD 0.14 billion in 2023 to USD 0.26 billion by 2033. Increasing adoption of advanced healthcare technologies and a growing number of interventions are anticipated to fuel market growth, alongside support from government initiatives encouraging healthcare advancements.Middle East & Africa Radioactive Stents Market Report:
In the Middle East and Africa, the market for Radioactive Stents is projected to rise from USD 0.09 billion in 2023 to USD 0.16 billion by 2033, driven by increasing healthcare investment and growing awareness of advanced treatment options among patients and healthcare professionals.Tell us your focus area and get a customized research report.
Radioactive Stents Market Analysis By Type
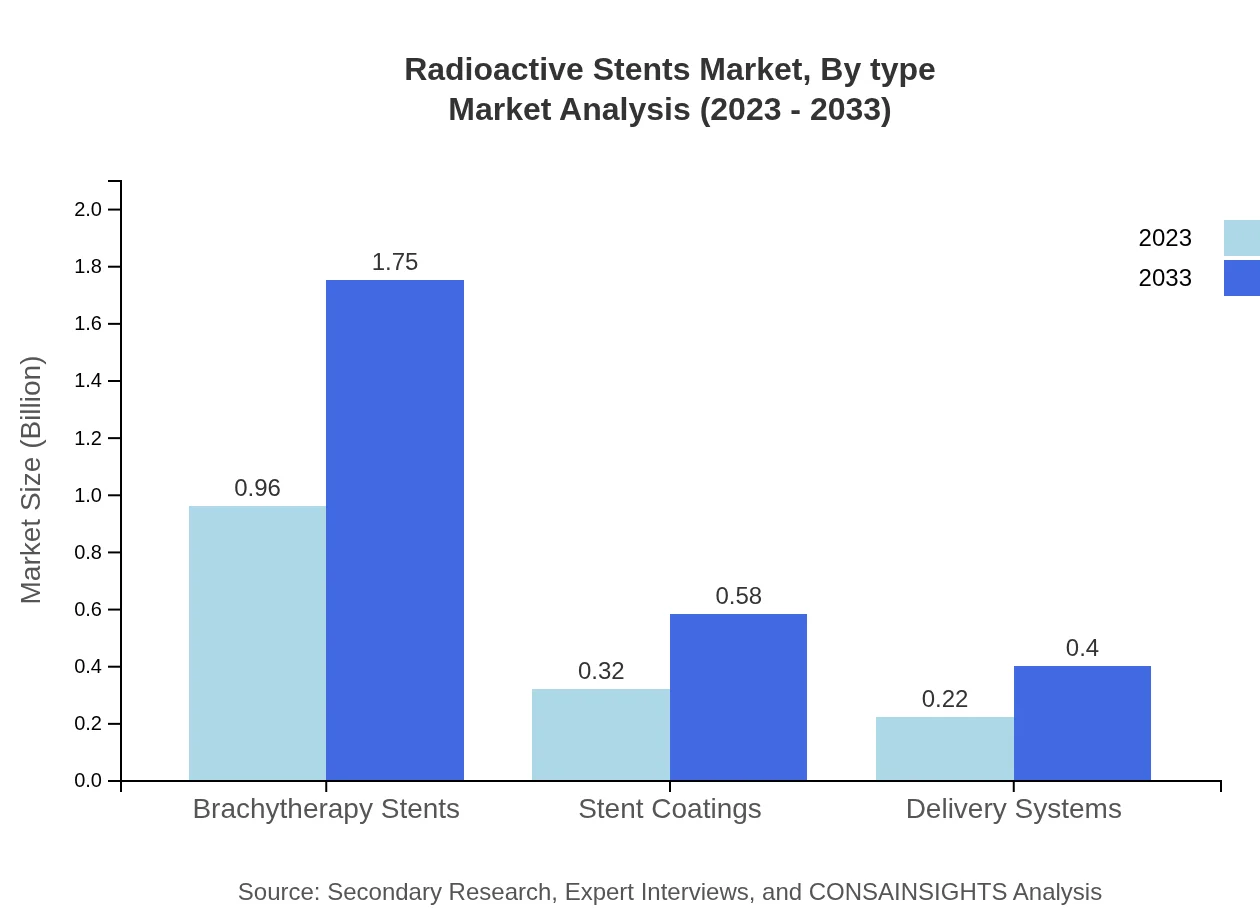
The market analysis by type reveals that Brachytherapy Stents dominate with a market size of USD 0.96 billion in 2023 and expected growth to USD 1.75 billion by 2033, capturing 64.19% market share. Stent coatings follow, holding a 21.11% share with market values moving from USD 0.32 billion to USD 0.58 billion over the same period. Delivery systems account for 14.7% of the market, increasing from USD 0.22 billion to USD 0.40 billion.
Radioactive Stents Market Analysis By Applications
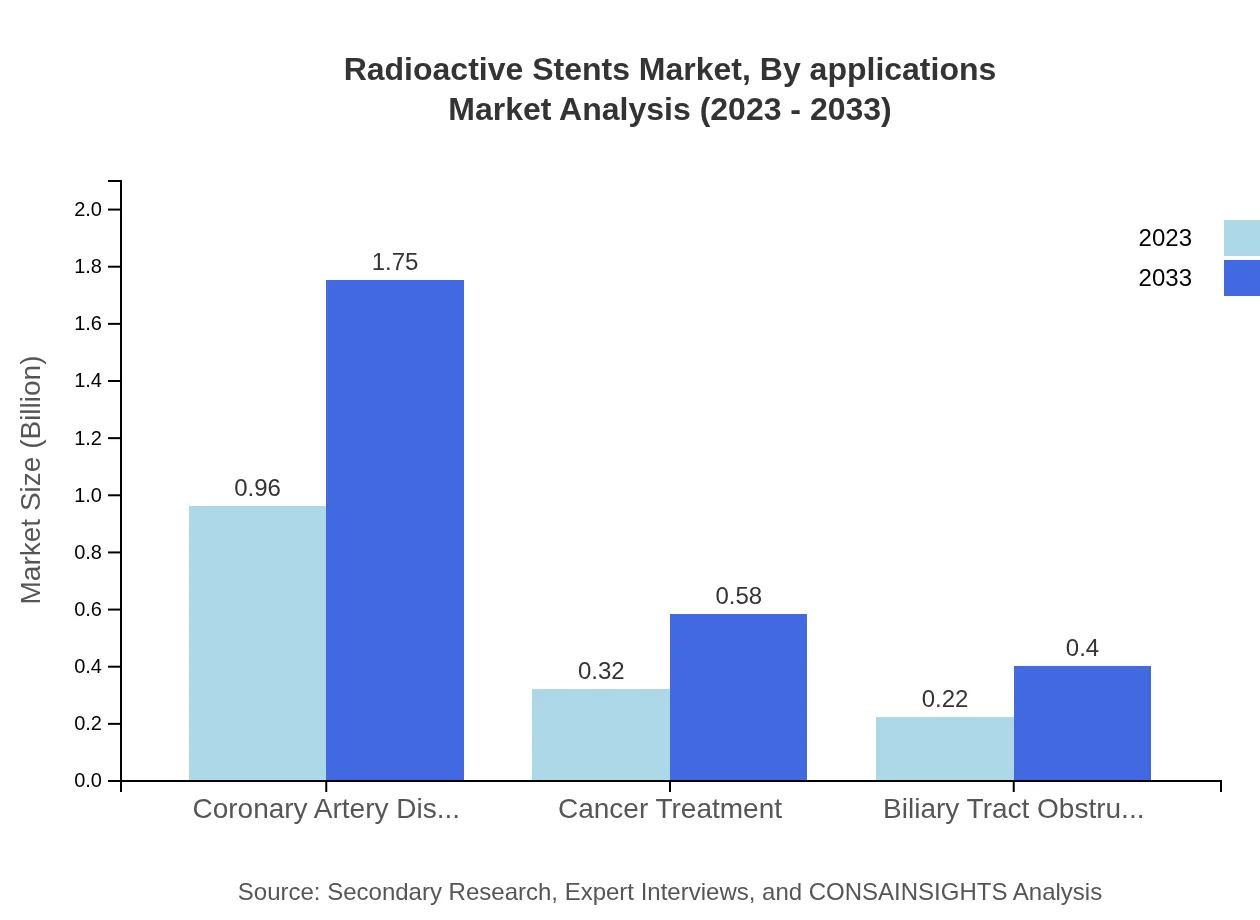
In terms of applications, the treatment of Coronary Artery Disease leads, with a market size of USD 0.96 billion in 2023 and estimated growth to USD 1.75 billion by 2033. This is followed by applications in Cancer Treatment, moving from USD 0.32 billion to USD 0.58 billion, representing 21.11% market share, and Biliary Tract Obstructions, scaling from USD 0.22 billion to USD 0.40 billion, capturing 14.7% of the market.
Radioactive Stents Market Analysis By End User
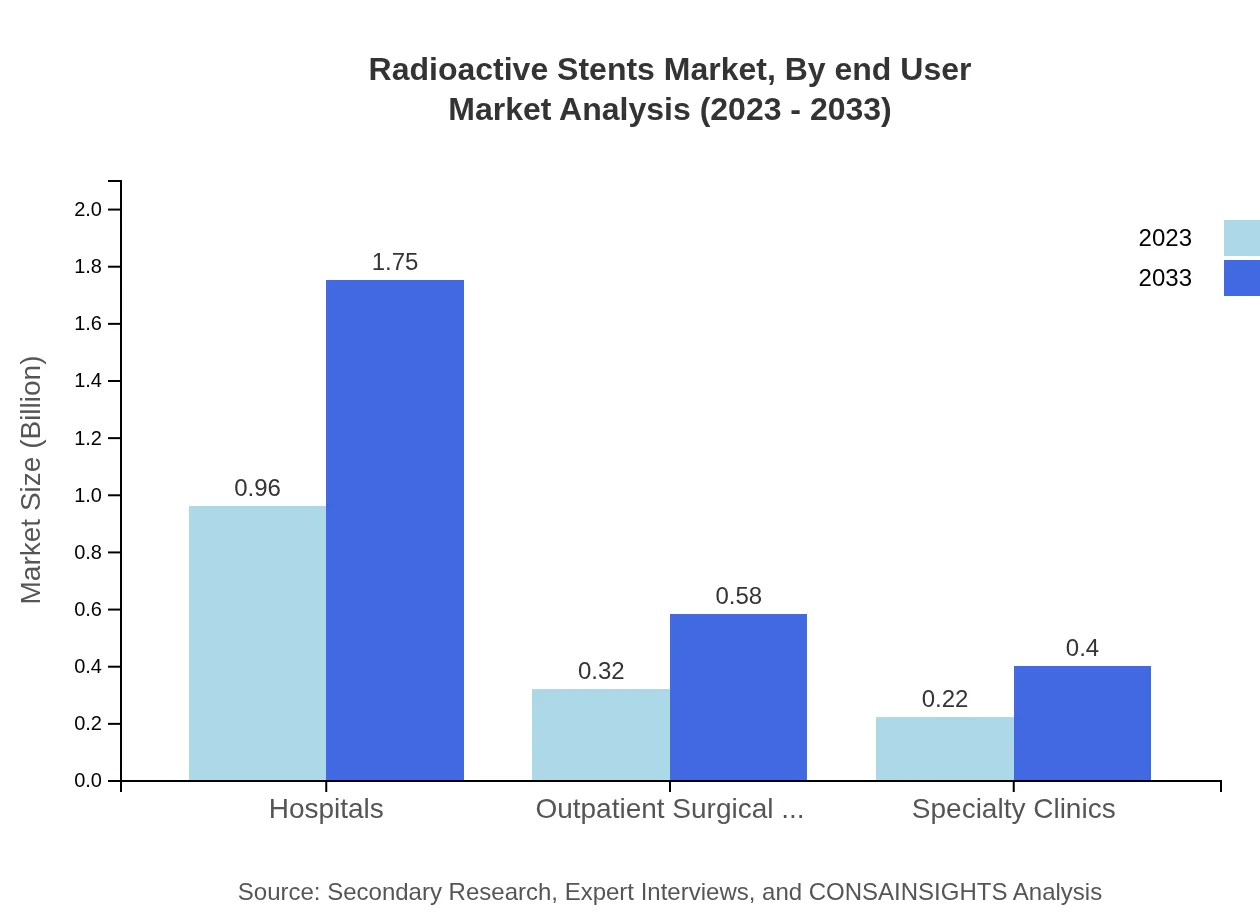
Hospitals lead the end-user market, with a size of USD 0.96 billion in 2023, growing to USD 1.75 billion by 2033, maintaining a steady share of 64.19%. Outpatient Surgical Centers cover 21.11% of the market and are projected to grow from USD 0.32 billion to USD 0.58 billion. Specialty Clinics contribute an additional 14.7%, growing from USD 0.22 billion to USD 0.40 billion.
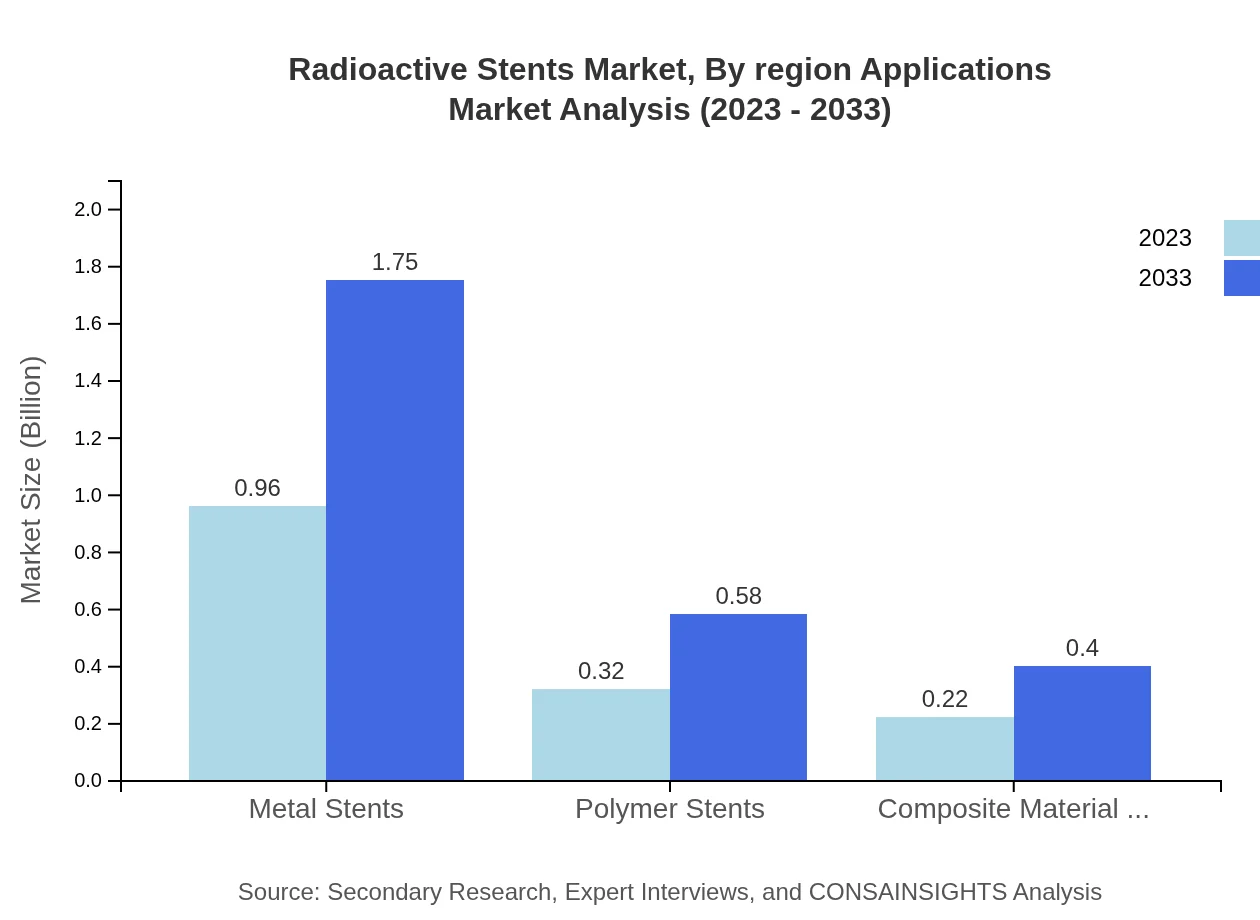
Radioactive Stents Market Analysis By Region Applications
The composition of the Radioactive Stents market by material reveals that Metal Stents lead in performance, accounting for USD 0.96 billion in 2023 and expected to reach USD 1.75 billion by 2033 with 64.19% share. Polymer Stents represent 21.11% of the market, moving from USD 0.32 billion to USD 0.58 billion. Composite Material Stents cover 14.7% share, growing from USD 0.22 billion to USD 0.40 billion.
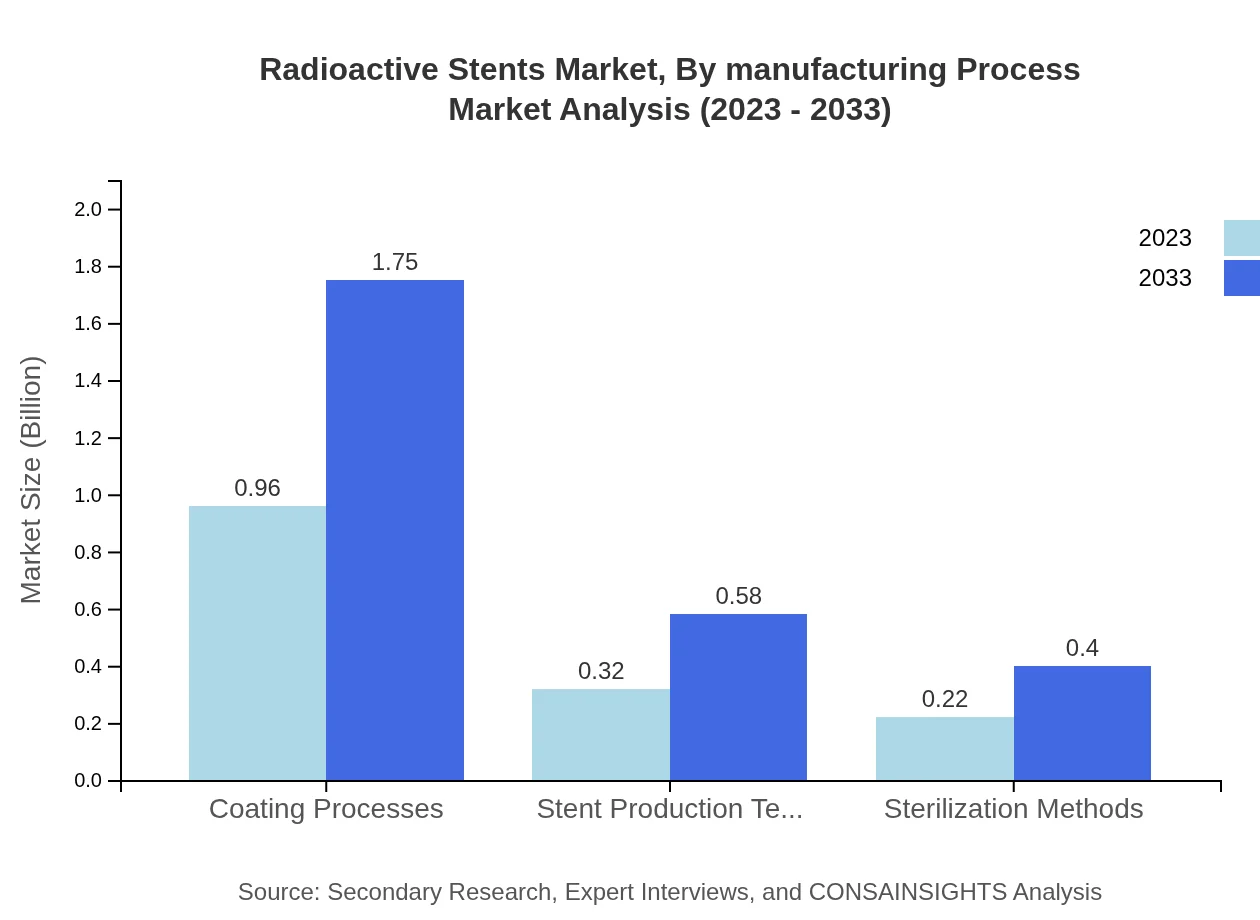
Radioactive Stents Market Analysis By Manufacturing Process
In manufacturing processes, Coating Processes lead with a market size of USD 0.96 billion in 2023, expected to grow to USD 1.75 billion by 2033, holding 64.19% share. Stent Production Techniques account for 21.11% and are projected to grow from USD 0.32 billion to USD 0.58 billion, while Sterilization Methods will capture 14.7% market share, increasing from USD 0.22 billion to USD 0.40 billion.
Radioactive Stents Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Radioactive Stents Industry
Boston Scientific Corporation:
Boston Scientific is a global leader in the development of innovative medical devices, including advanced stents. Their significant research focus on radioactive stents aligns with advancements in cardiovascular therapies.Abbott Laboratories:
Abbott Laboratories is known for its comprehensive healthcare products, particularly in cardiovascular devices. Their contributions to medication-coated stents have influenced the development of radioactive stent technology.Medtronic :
Medtronic is a pioneer in the medical technology sector, particularly known for its cardiac devices. Their ongoing investments into R&D for stent technologies reflect their commitment to improving patient outcomes.Bard Peripheral Vascular:
Bard specializes in vascular medical devices, contributing significantly to advancements in stent technologies, including innovative radioactive stent applications for numerous therapeutic procedures.Stryker Corporation:
With a strong specialization in orthopedic and medical devices, Stryker's involvement in vascular interventions includes development and distribution of advanced stenting solutions.We're grateful to work with incredible clients.









FAQs
What is the market size of radioactive stents?
The global market size for radioactive stents is projected at $1.5 billion in 2023, with a compound annual growth rate (CAGR) of 6.0% expected through 2033, indicating steady growth in adoption and technological advancements.
What are the key market players or companies in the radioactive stents industry?
Key players in the radioactive stents industry include major medical device manufacturers and innovative startups. These companies are involved in research, development, and the commercialization of stent technologies that incorporate radioactive materials for enhanced therapeutic effects.
What are the primary factors driving the growth in the radioactive stents industry?
Growth drivers in the radioactive stents market include rising incidences of coronary artery diseases and cancer, advancements in stent technologies, regulatory approvals for new products, and the increasing prevalence of minimally invasive procedures in healthcare.
Which region is the fastest Growing in the radioactive stents?
The fastest-growing region for radioactive stents is North America, projected to grow from $0.55 billion in 2023 to $0.99 billion by 2033, driven by advanced healthcare infrastructure and increased adoption of innovative stent technologies.
Does ConsaInsights provide customized market report data for the radioactive stents industry?
Yes, ConsaInsights offers customized market report data, tailored to the specific needs of clients within the radioactive stents industry. This includes analysis of market trends, competitive landscape, and regional insights for informed decision-making.
What deliverables can I expect from this radioactive stents market research project?
Deliverables from the market research project include detailed market reports, insights into key segments, competitive analysis, regional growth forecasts, and actionable recommendations based on thorough market studies.
What are the market trends of radioactive stents?
Current market trends for radioactive stents include increasing use of brachytherapy technologies, growing patient populations suffering from cardiovascular and oncological conditions, and rising demand for innovative delivery systems, reflecting advancements in medical technology.