Rapid Diagnostic Kits Market Report
Published Date: 31 January 2026 | Report Code: rapid-diagnostic-kits
Rapid Diagnostic Kits Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Rapid Diagnostic Kits market, covering market size, growth forecasts for 2023-2033, segmentation, regional insights, and future trends impacting the industry. The report aims to equip stakeholders with valuable insights and informative data.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
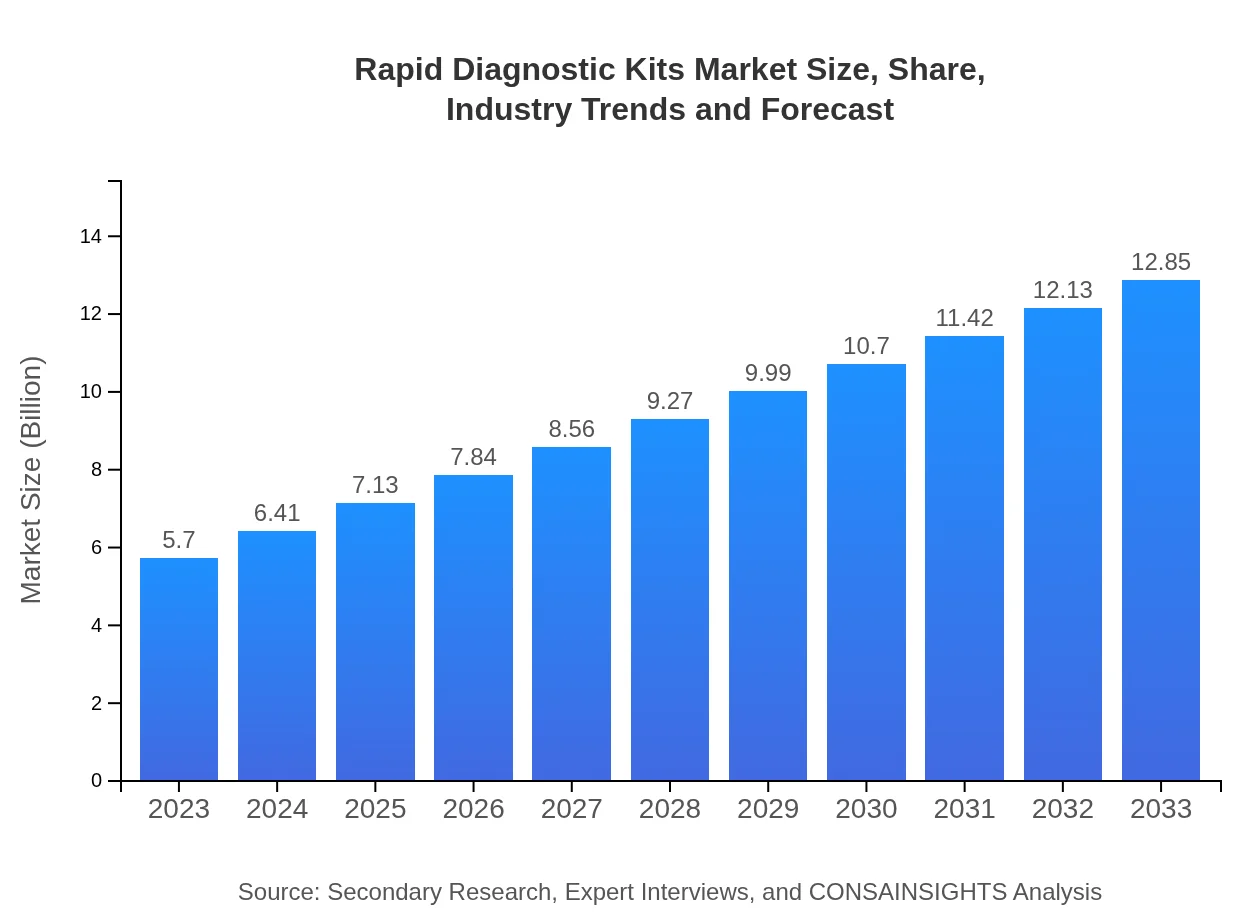
| 2023 Market Size | $5.70 Billion |
| CAGR (2023-2033) | 8.2% |
| 2033 Market Size | $12.85 Billion |
| Top Companies | Abbott Laboratories, Roche Diagnostics, Siemens Healthineers, Thermo Fisher Scientific |
| Last Modified Date | 31 January 2026 |
Rapid Diagnostic Kits Market Overview
Customize Rapid Diagnostic Kits Market Report market research report
- ✔ Get in-depth analysis of Rapid Diagnostic Kits market size, growth, and forecasts.
- ✔ Understand Rapid Diagnostic Kits's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Rapid Diagnostic Kits
What is the Market Size & CAGR of Rapid Diagnostic Kits market in 2023?
Rapid Diagnostic Kits Industry Analysis
Rapid Diagnostic Kits Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Rapid Diagnostic Kits Market Analysis Report by Region
Europe Rapid Diagnostic Kits Market Report:
Europe's market is anticipated to grow from USD 1.56 billion in 2023 to USD 3.52 billion by 2033, with key advancements in biotechnology and partnerships between healthcare providers and diagnostic companies boosting the adoption of rapid diagnostic tests.Asia Pacific Rapid Diagnostic Kits Market Report:
In the Asia Pacific region, the Rapid Diagnostic Kits market is estimated at USD 1.10 billion in 2023 and expected to reach USD 2.48 billion by 2033. The growth is attributed to increasing healthcare expenditure and rising awareness regarding infectious diseases, alongside substantial investments in healthcare infrastructure.North America Rapid Diagnostic Kits Market Report:
North America dominates the Rapid Diagnostic Kits market, projected at USD 2.00 billion in 2023, expected to grow to USD 4.52 billion by 2033. The rise in health awareness, alongside the growing trend of home testing and point-of-care diagnostics, significantly contributes to this growth.South America Rapid Diagnostic Kits Market Report:
The South American market for Rapid Diagnostic Kits is projected to grow from USD 0.26 billion in 2023 to USD 0.58 billion by 2033. Market growth is driven by improvements in healthcare accessibility and increasing public health initiatives being implemented by governments across various countries.Middle East & Africa Rapid Diagnostic Kits Market Report:
The Middle East and Africa market is expected to increase from USD 0.77 billion in 2023 to USD 1.74 billion by 2033, fueled by enhanced healthcare access and growing investments in healthcare innovations aiming to improve diagnostic services.Tell us your focus area and get a customized research report.
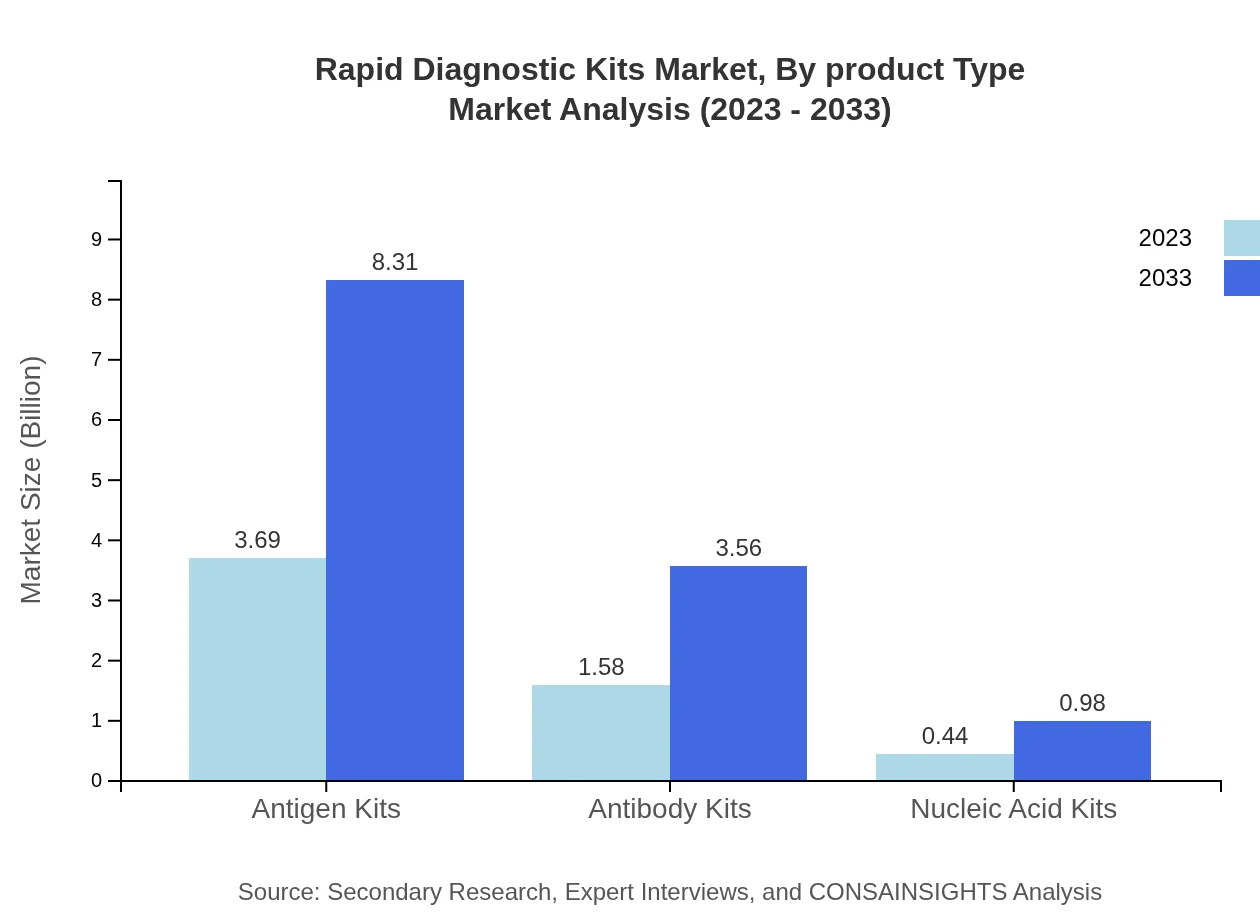
Rapid Diagnostic Kits Market Analysis By Product Type
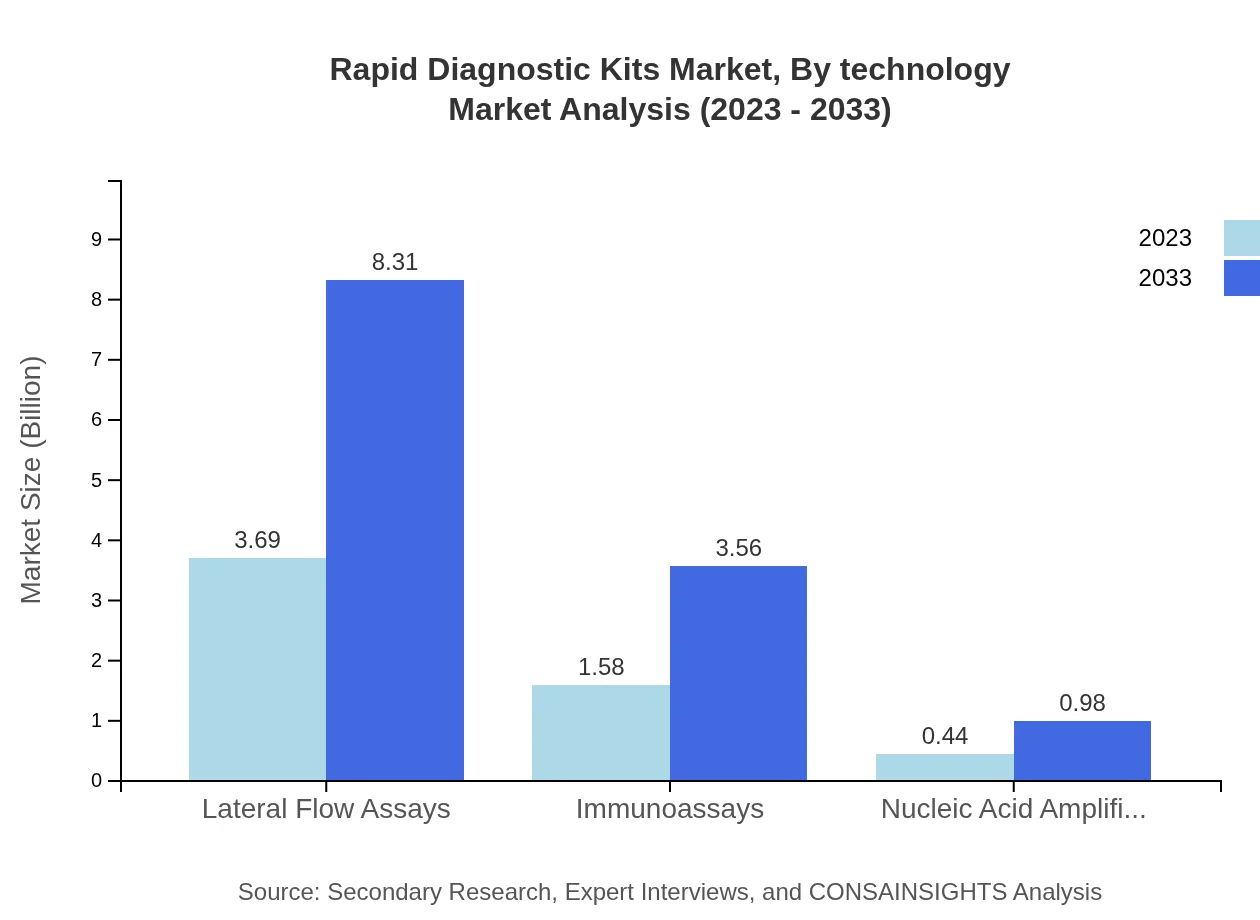
Lateral Flow Assays dominate the Rapid Diagnostic Kits market in both size and share. In 2023, this segment is valued at USD 3.69 billion with a market share of 64.66% and is projected to reach USD 8.31 billion by 2033. Immunoassays follow closely, holding a significant market size of USD 1.58 billion (27.68% share) in 2023, with estimates suggesting it could grow to USD 3.56 billion by 2033. Nucleic Acid Amplification Tests, although smaller in market size, show promising growth with an increase from USD 0.44 billion to USD 0.98 billion during the same period?
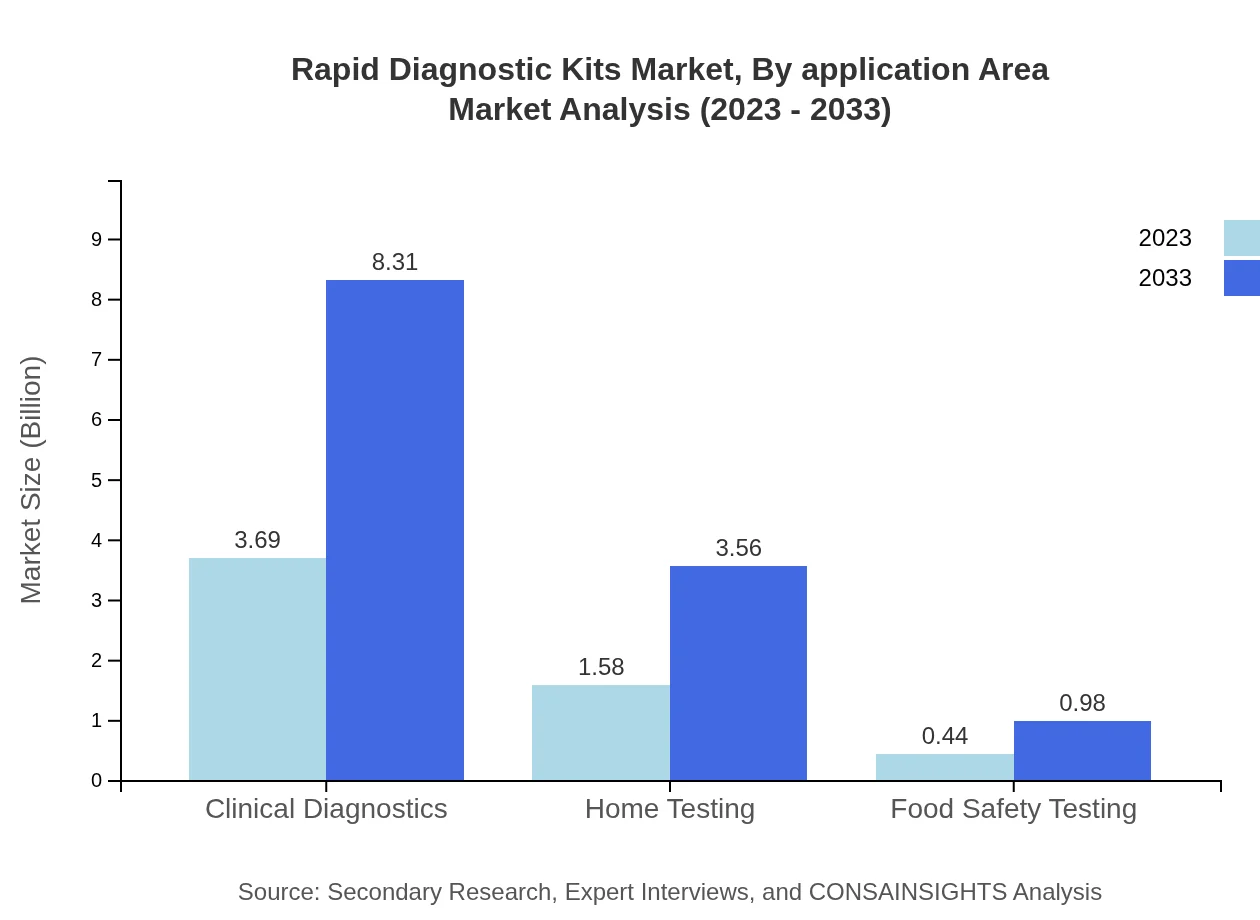
Rapid Diagnostic Kits Market Analysis By Application Area
The Clinical Diagnostics sector leads the Rapid Diagnostic Kits market, accounting for USD 3.69 billion (64.66% share) in 2023, anticipated to expand to USD 8.31 billion by 2033. Home Testing also plays a pivotal role, projected to grow from USD 1.58 billion (27.68% share) to USD 3.56 billion over the period. This reflects a growing consumer shift towards at-home testing solutions.
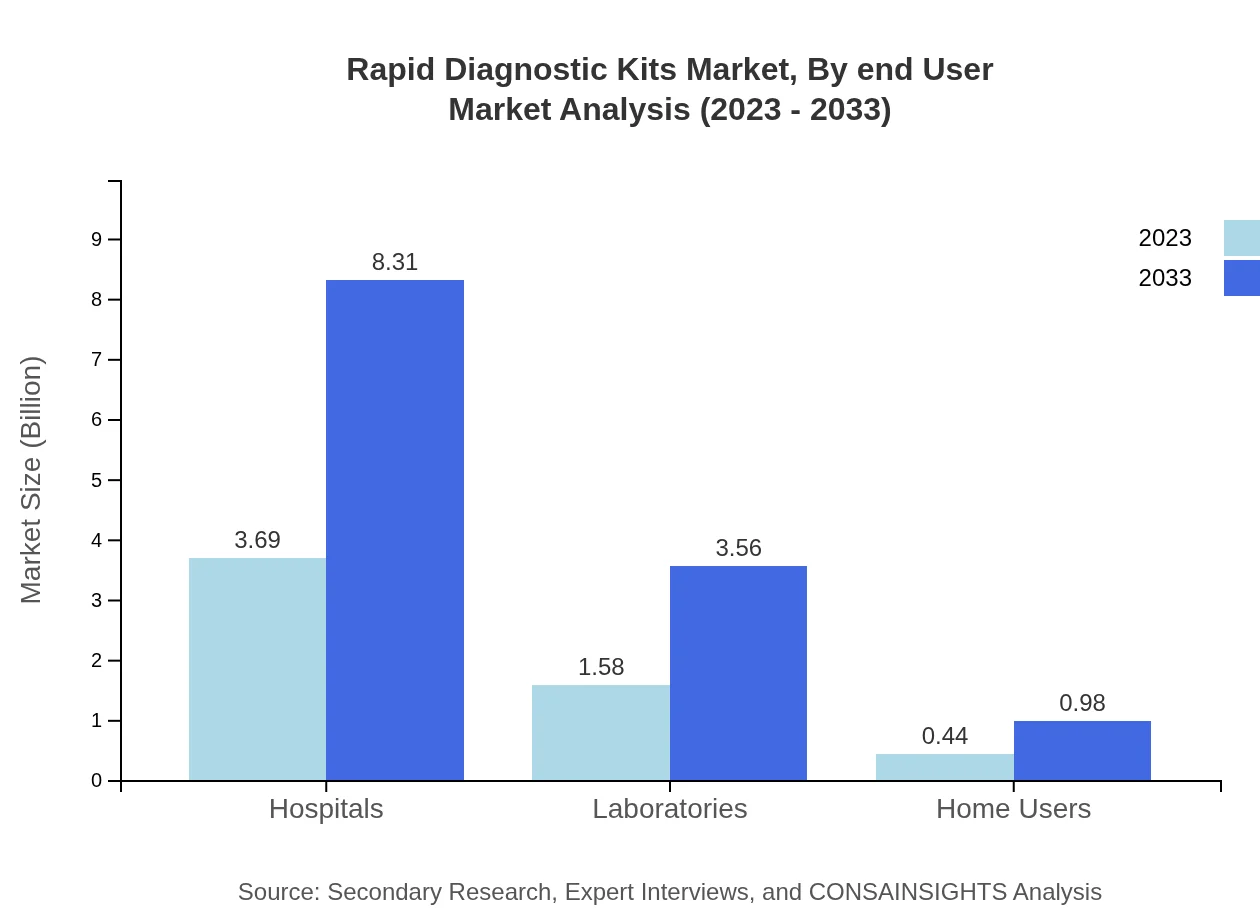
Rapid Diagnostic Kits Market Analysis By End User
Hospitals are the primary end-users of Rapid Diagnostic Kits, with the segment expected to grow from USD 3.69 billion in 2023 to USD 8.31 billion by 2033, maintaining a dominant market share of 64.66%. Laboratories and home users also contribute significantly, while home users are expected to see substantial growth, reflecting the increasing trend towards direct consumer engagement in healthcare.
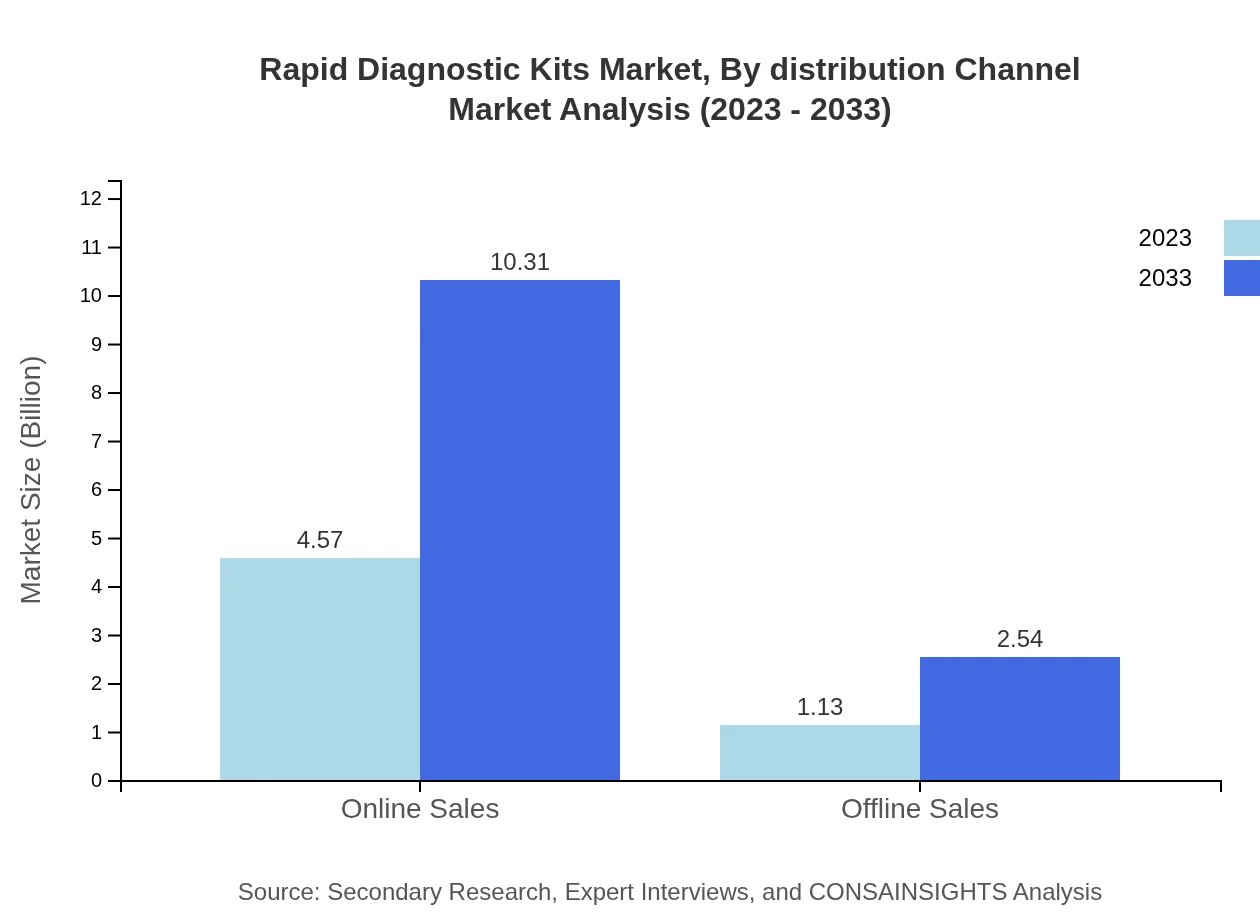
Rapid Diagnostic Kits Market Analysis By Distribution Channel
The distribution landscape for Rapid Diagnostic Kits is heavily skewed towards online sales, anticipated to dominate with a market size of USD 4.57 billion (80.23%) in 2023, growing to USD 10.31 billion by 2033. Offline channels, while significantly smaller, are projected to grow from USD 1.13 billion to USD 2.54 billion, highlighting the importance of diverse distribution strategies.
Rapid Diagnostic Kits Market Analysis By Technology
Technological advancements are a key driver in the Rapid Diagnostic Kits industry. Enhanced sensitivity and specificity in tests, simplified operations, and integration with IT platforms for data management are vital trends. Innovations like mobile applications complement these kits, providing comprehensive testing solutions directly to consumers.
Rapid Diagnostic Kits Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Rapid Diagnostic Kits Industry
Abbott Laboratories:
Abbott is a leader in rapid point-of-care testing and has developed numerous diagnostics solutions that span various diseases, leveraging state-of-the-art technology for health improvement.Roche Diagnostics:
Roche is renowned for its innovative diagnostics solutions and continues to evolve in the Rapid Diagnostic Kits segment, bringing advanced testing technologies to market.Siemens Healthineers:
Siemens offers a wide array of diagnostic kits and has a strong focus on enhancing healthcare efficiency through its diagnostic solutions.Thermo Fisher Scientific:
Thermo Fisher provides a range of high-quality diagnostic kits that aid in research and clinical diagnostics, catering to both professional and home users.We're grateful to work with incredible clients.









FAQs
What is the market size of Rapid Diagnostic Kits?
The global market size for Rapid Diagnostic Kits is projected to reach approximately $5.7 billion by 2033, with a compound annual growth rate (CAGR) of 8.2%. This growth reflects increasing demand for quick and accurate testing solutions.
What are the key market players or companies in this Rapid Diagnostic Kits industry?
Key players in the Rapid Diagnostic Kits industry include major healthcare giants and specialized diagnostics firms. These companies focus on innovation, quality, and expanding their product offerings to meet rising global healthcare demands.
What are the primary factors driving the growth in the Rapid Diagnostic Kits industry?
Growth in the Rapid Diagnostic Kits industry is primarily driven by an increasing prevalence of infectious diseases, growing consumer awareness, technological advancements, and the rising demand for point-of-care testing solutions.
Which region is the fastest Growing in the Rapid Diagnostic Kits?
The fastest-growing region for Rapid Diagnostic Kits is North America, where the market size is projected to grow from $2.00 billion in 2023 to $4.52 billion by 2033, driven by technological advancements and increased healthcare spending.
Does Consainsights provide customized market report data for the Rapid Diagnostic Kits industry?
Yes, Consainsights offers customized market report data tailored to specific interests and needs within the Rapid Diagnostic Kits industry, ensuring clients receive relevant insights for informed decision-making.
What deliverables can I expect from this Rapid Diagnostic Kits market research project?
Deliverables from the Rapid Diagnostic Kits market research project typically include comprehensive reports, detailed market analysis, forecasting data, competitive landscape evaluations, and strategic recommendations tailored to your organizational needs.
What are the market trends of Rapid Diagnostic Kits?
Current market trends in Rapid Diagnostic Kits include a shift towards home testing, technological advancements in diagnostic methods, increased online sales, and a focus on rapid testing solutions to respond to health emergencies.