Rapid Influenza Diagnostic Test Ridt Market Report
Published Date: 31 January 2026 | Report Code: rapid-influenza-diagnostic-test-ridt
Rapid Influenza Diagnostic Test Ridt Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Rapid Influenza Diagnostic Test (RIDT) market from 2023 to 2033, covering key insights, data trends, market dynamics, and forecasts that cater to stakeholders in the healthcare industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
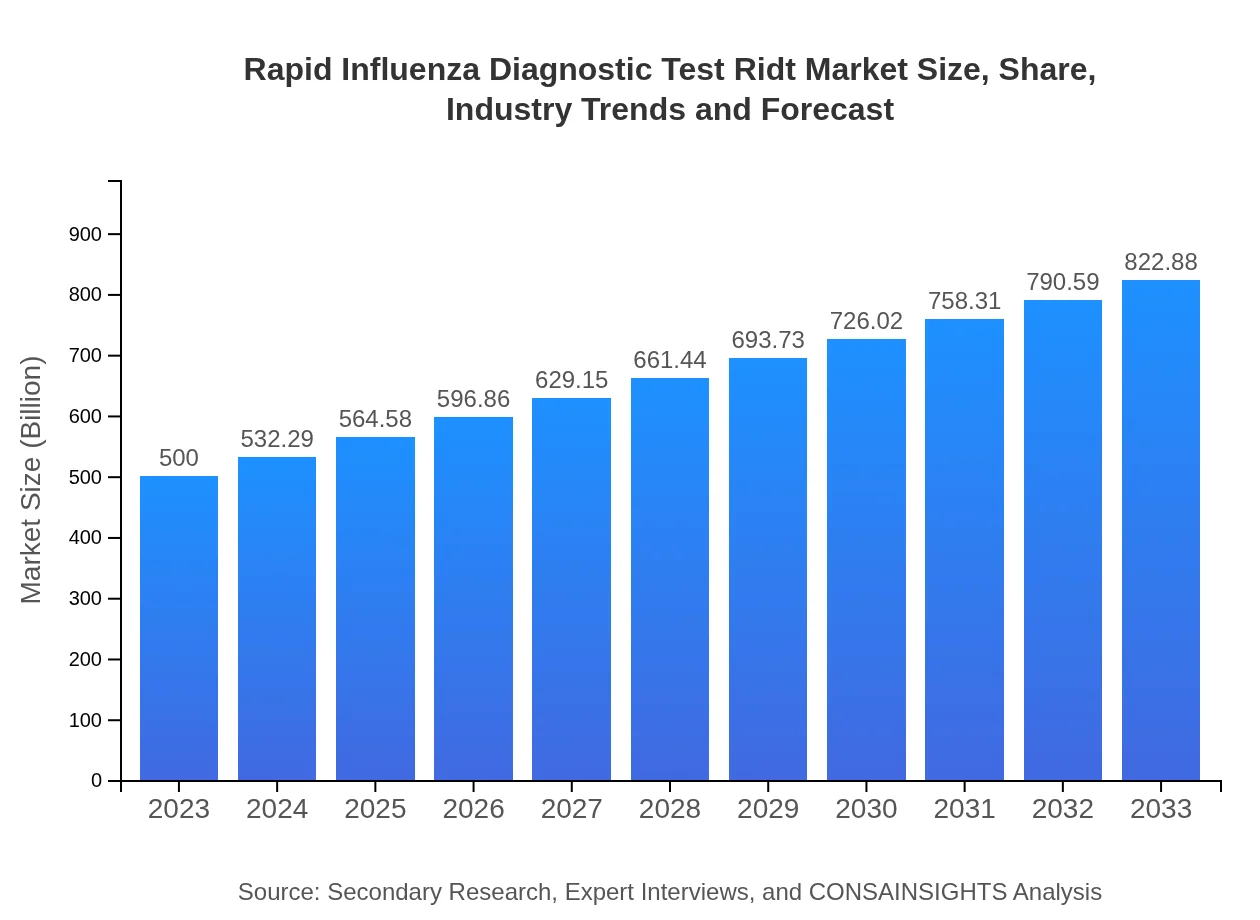
| 2023 Market Size | $500.00 Million |
| CAGR (2023-2033) | 5% |
| 2033 Market Size | $822.88 Million |
| Top Companies | Abbott Laboratories, Roche Diagnostics, Quidel Corporation, BD (Becton, Dickinson and Company) |
| Last Modified Date | 31 January 2026 |
Rapid Influenza Diagnostic Test Ridt Market Overview
Customize Rapid Influenza Diagnostic Test Ridt Market Report market research report
- ✔ Get in-depth analysis of Rapid Influenza Diagnostic Test Ridt market size, growth, and forecasts.
- ✔ Understand Rapid Influenza Diagnostic Test Ridt's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Rapid Influenza Diagnostic Test Ridt
What is the Market Size & CAGR of Rapid Influenza Diagnostic Test Ridt market in 2023?
Rapid Influenza Diagnostic Test Ridt Industry Analysis
Rapid Influenza Diagnostic Test Ridt Market Segmentation and Scope
Tell us your focus area and get a customized research report.
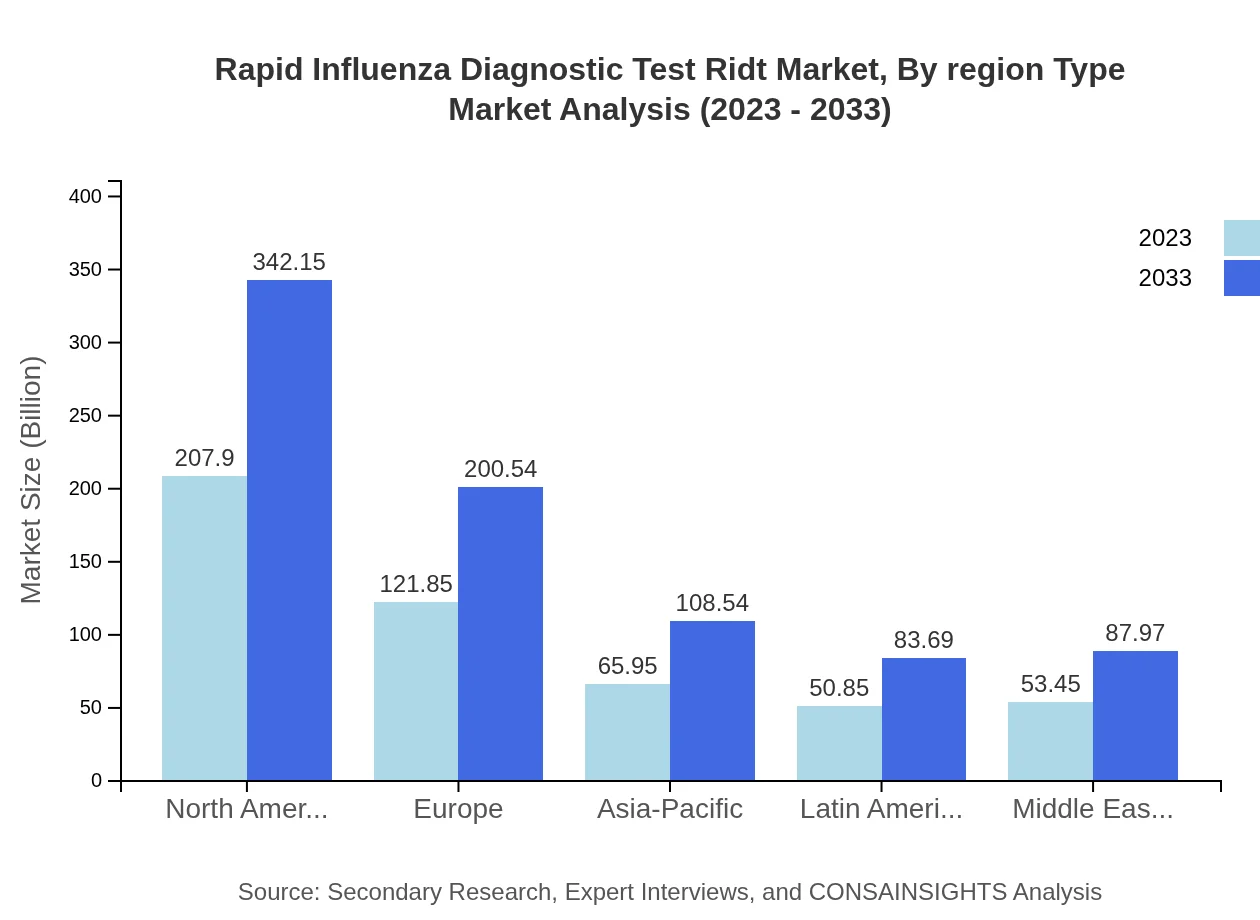
Rapid Influenza Diagnostic Test Ridt Market Analysis Report by Region
Europe Rapid Influenza Diagnostic Test Ridt Market Report:
Europe, currently valued at $130.70 million in 2023, is forecasted to reach $215.10 million by 2033, propelled by a beneficial regulatory environment and advancements in healthcare technologies.Asia Pacific Rapid Influenza Diagnostic Test Ridt Market Report:
Asian countries like Japan and China are increasingly adopting RIDTs due to their significant population and high influenza infection rates. In 2023, the market size is approximately $97.95 million and is expected to reach $161.20 million by 2033, benefiting from rising healthcare investments and technological advancements.North America Rapid Influenza Diagnostic Test Ridt Market Report:
North America is a leading region in the RIDT market, with a market size of $194.10 million in 2023, projected to increase to $319.44 million by 2033. This growth is attributed to high awareness about influenza diagnostics and robust healthcare infrastructure.South America Rapid Influenza Diagnostic Test Ridt Market Report:
The South American RIDT market, valued at $14.95 million in 2023, is expected to grow to $24.60 million by 2033. The expansion is driven by increasing prevalence of influenza and improving access to healthcare services.Middle East & Africa Rapid Influenza Diagnostic Test Ridt Market Report:
The Middle East and Africa corridors show potential for growth, starting at $62.30 million in 2023 and projected to hit $102.53 million by 2033 as access to healthcare and diagnostic technologies improve in these regions.Tell us your focus area and get a customized research report.
Rapid Influenza Diagnostic Test Ridt Market Analysis By Product Type
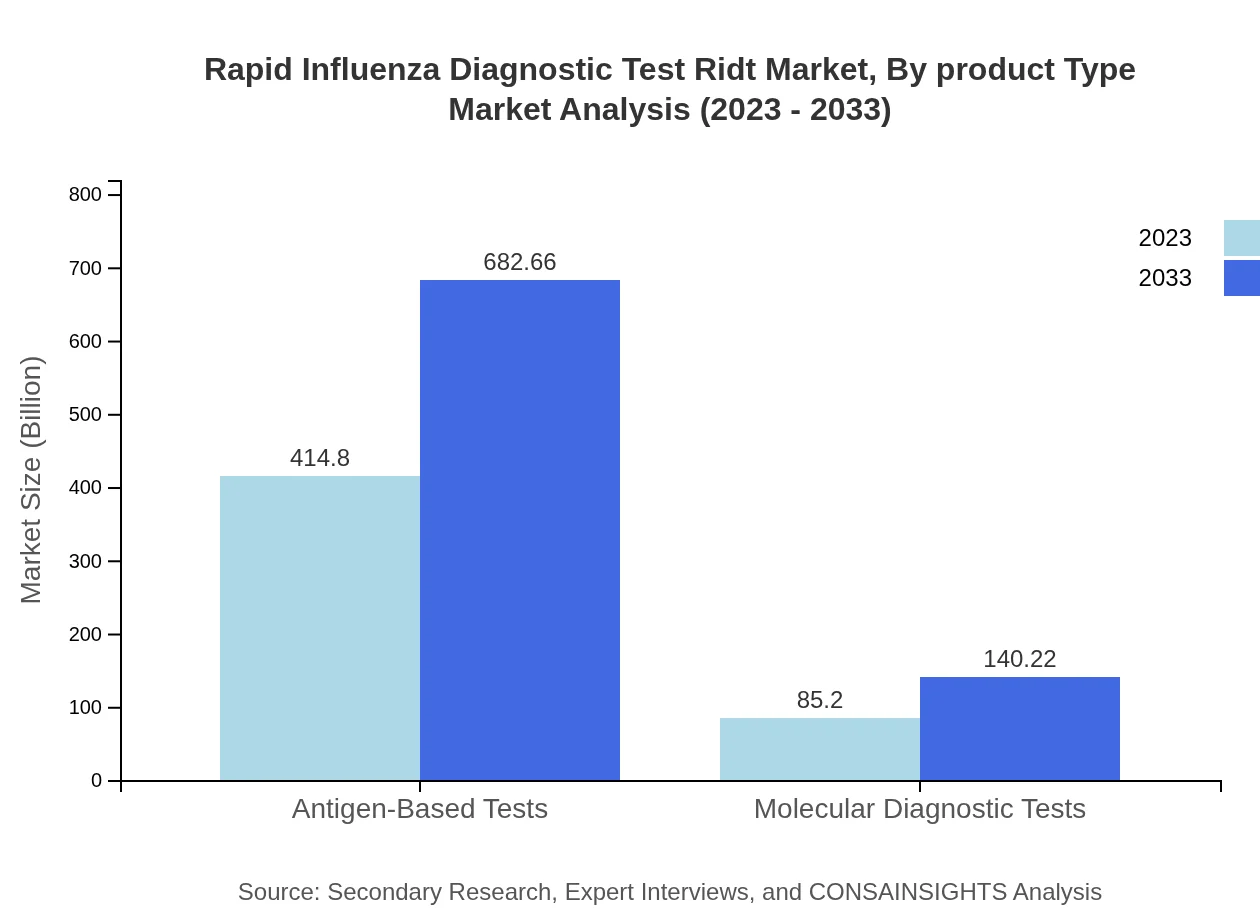
The product types are categorized primarily into antigen-based tests and nucleic acid amplification tests (NAAT), with antigen tests dominating the market due to their ease of use and rapid results. In 2023, antigen-based tests comprises $414.80 million of the RIDT market, while NAAT captures $85.20 million. Over the next decade, innovation in molecular diagnostics is expected to drive the growth of NAAT significantly.
Rapid Influenza Diagnostic Test Ridt Market Analysis By End User
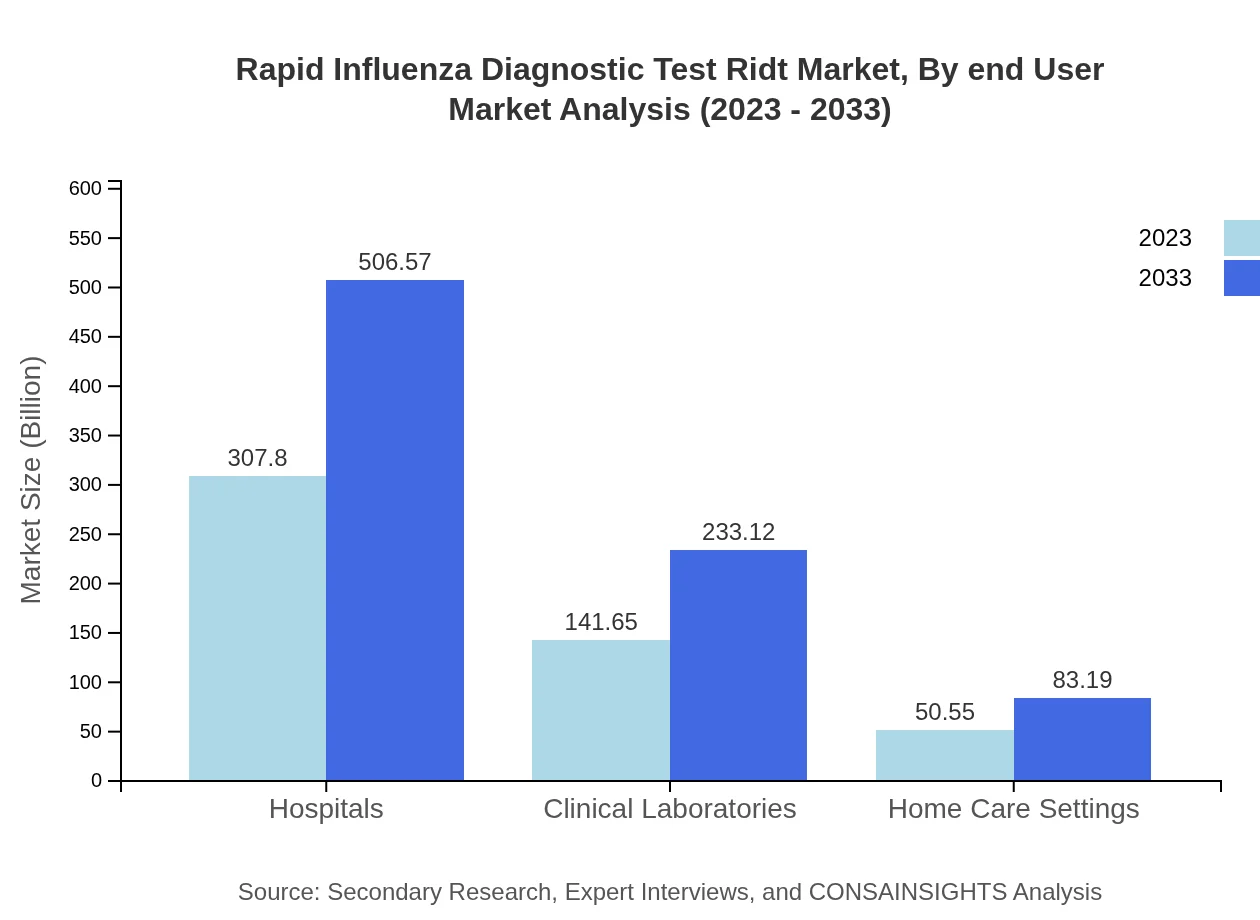
The RIDT market is majorly segmented into hospitals, clinical laboratories, home care settings, pharmacies, and online retail. Hospitals lead with approximately $307.80 million market share in 2023, bolstered by high patient inflow and diagnostic demands. Clinical laboratories follow with $141.65 million, while home care settings represent a growing segment valued at $50.55 million, showcasing the trend towards at-home testing.
Rapid Influenza Diagnostic Test Ridt Market Analysis By Technology
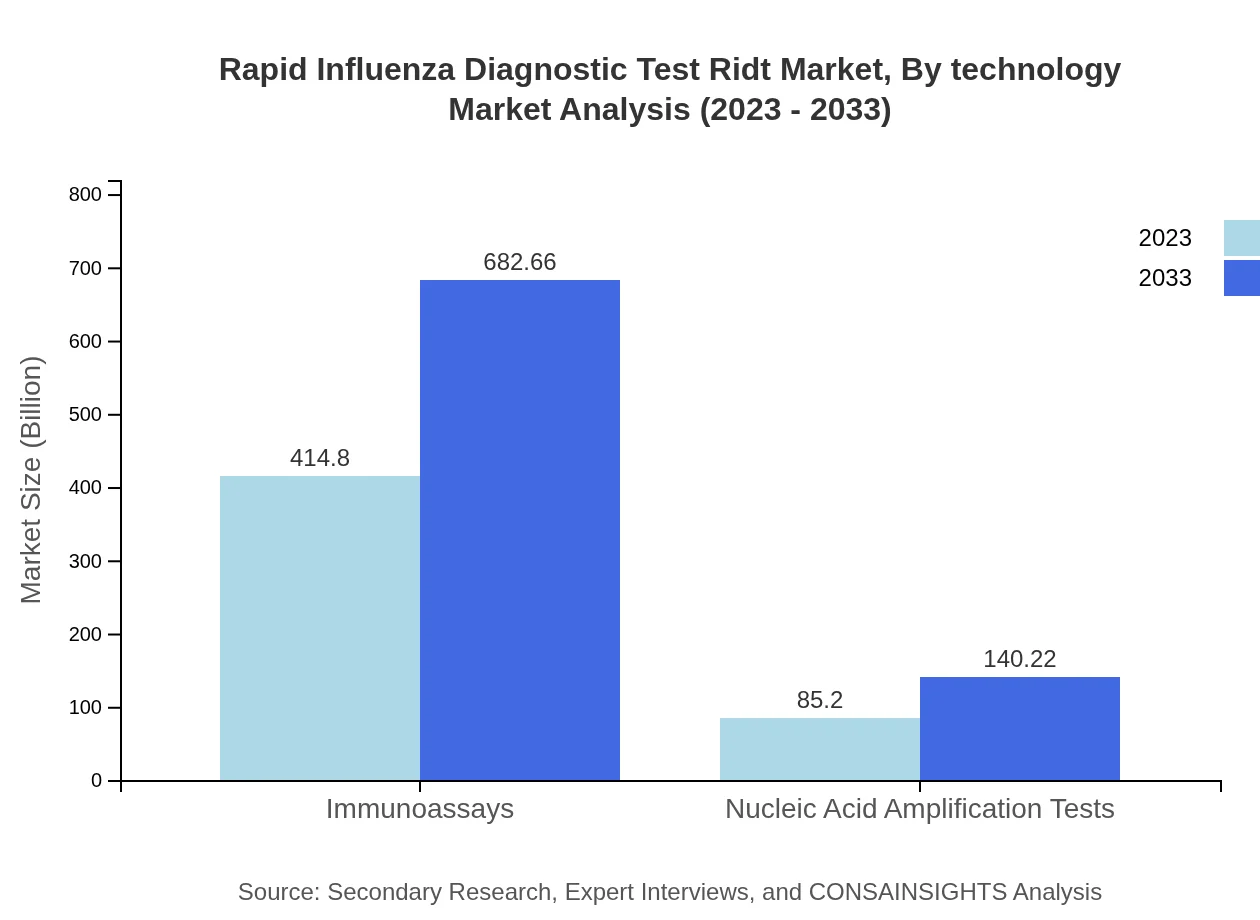
Technological advancements in the RIDT market are centered around antigen and nucleic acid amplification tests. Antigen-based tests dominate with a market share of $414.80 million in 2023, primarily due to their rapid turnaround time. Conversely, molecular diagnostic tests are anticipated to grow from $85.20 million and gain traction from healthcare providers looking for accuracy.
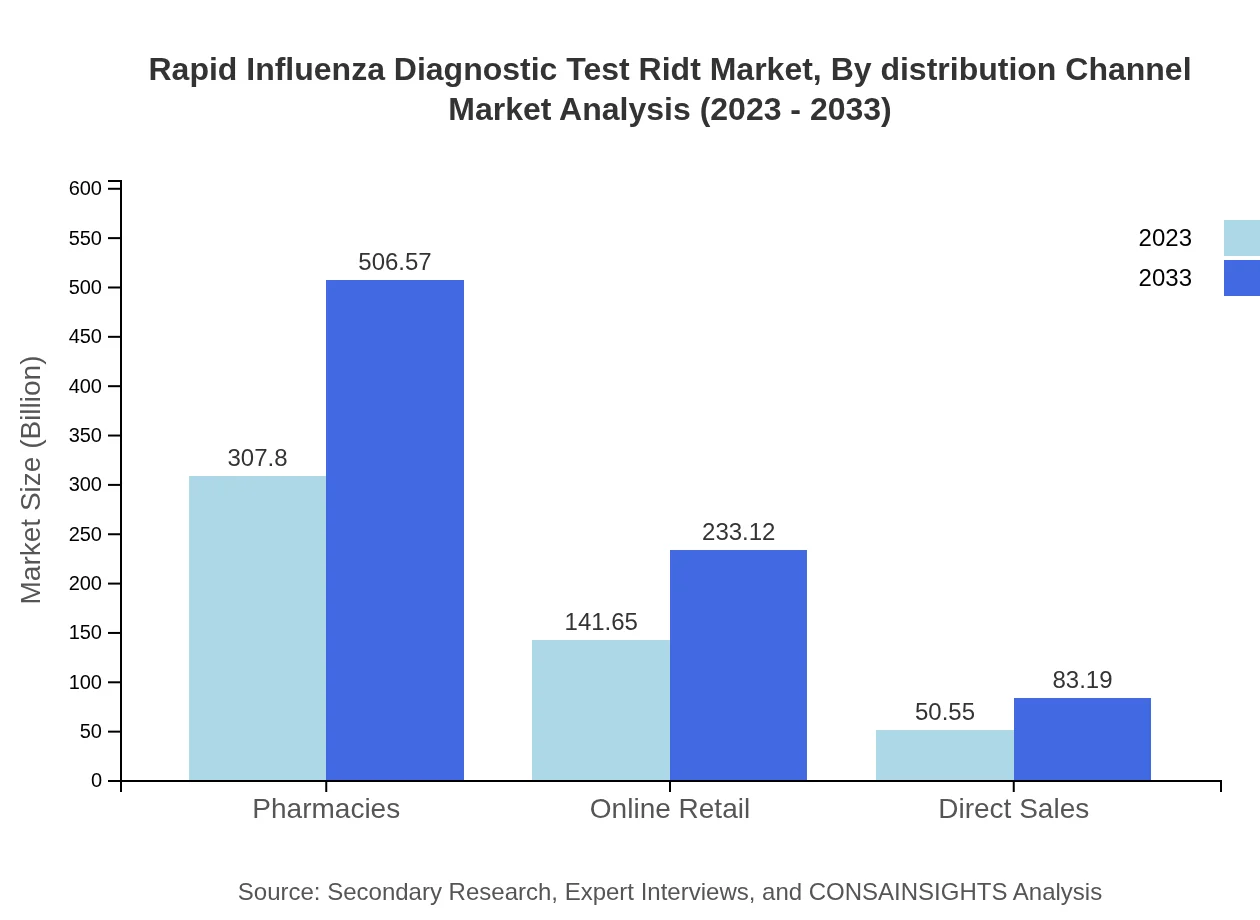
Rapid Influenza Diagnostic Test Ridt Market Analysis By Distribution Channel
The distribution channels of RIDTs range from direct sales to online retail. Direct sales are forecasted to generate $50.55 million in 2023, whilst pharmacies and online retail channels capture significant shares, valued at $307.80 million and $141.65 million respectively, showcasing an increasing trend towards digital healthcare solutions.
Rapid Influenza Diagnostic Test Ridt Market Analysis By Region Type
Regionally, North America leads the RIDT market, supported by technological advancements and a strong healthcare system. Europe and Asia-Pacific also show substantial market growth potential. The overall market dynamics suggest continuous regional improvements in healthcare accessibility and diagnostic technology adoption.
Rapid Influenza Diagnostic Test Ridt Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Rapid Influenza Diagnostic Test Ridt Industry
Abbott Laboratories:
A leading global healthcare company involved in the development of cutting-edge rapid diagnostic tests, including RIDTs, offering a wide array of influenza testing solutions.Roche Diagnostics:
A major player in the diagnostics industry, Roche develops innovative RIDTs contributing significantly to enhanced testing capabilities in hospitals and laboratories worldwide.Quidel Corporation:
Renowned for its antigen-based diagnostic solutions, Quidel provides reliable RIDT products that facilitate rapid and accurate detection of influenza viruses.BD (Becton, Dickinson and Company):
BD offers a broad range of diagnostic technologies, including RIDTs, enhancing the efficiency of healthcare services through innovative solutions.We're grateful to work with incredible clients.









FAQs
What is the market size of Rapid Influenza Diagnostic Test (RIDT)?
The global market size of Rapid Influenza Diagnostic Tests (RIDT) is projected to reach approximately $500 million in 2023, with an estimated CAGR of 5% over the next decade, indicating steady growth in demand for these diagnostic solutions.
What are the key market players or companies in the RIDT industry?
Key market players in the RIDT industry include major diagnostic companies which continually innovate to capture market share. Their competitive strategies focus on product development, strategic partnerships, and technological advancements to meet growing consumer and healthcare demands.
What are the primary factors driving the growth in the RIDT industry?
Growth in the RIDT industry is driven by factors such as rising respiratory infection incidences, technological advancements in diagnostic testing, increased awareness of flu-related diseases, and a growing preference for rapid testing solutions among healthcare professionals and patients.
Which region is the fastest Growing in the RIDT?
The fastest-growing region in the RIDT market is North America, projected to grow from $194.10 million in 2023 to $319.44 million by 2033. This growth is attributed to advanced healthcare infrastructure and increasing incidences of influenza.
Does ConsaInsights provide customized market report data for the RIDT industry?
Yes, ConsaInsights offers tailored market report data for the RIDT industry, allowing clients to access specific insights, market trends, and forecasts that align with their particular business strategies and objectives.
What deliverables can I expect from this RIDT market research project?
Deliverables from the RIDT market research project typically include detailed market analysis reports, trend forecasts, regional insights, and segmentation data, enabling informed strategic decisions and enhanced understanding of market dynamics.
What are the market trends of RIDT?
Current market trends in the RIDT sector include the increasing adoption of home testing kits, advancements in molecular diagnostic technologies, and a growing focus on rapid turnaround times for test results, all contributing to improved patient care outcomes.