Rapid Microbiology Testing Market Report
Published Date: 31 January 2026 | Report Code: rapid-microbiology-testing
Rapid Microbiology Testing Market Size, Share, Industry Trends and Forecast to 2033
This report delves into the global market for Rapid Microbiology Testing, providing insights into current trends, market size, and growth forecasts from 2023 to 2033. It analyzes regional dynamics and leading players within this expanding field.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
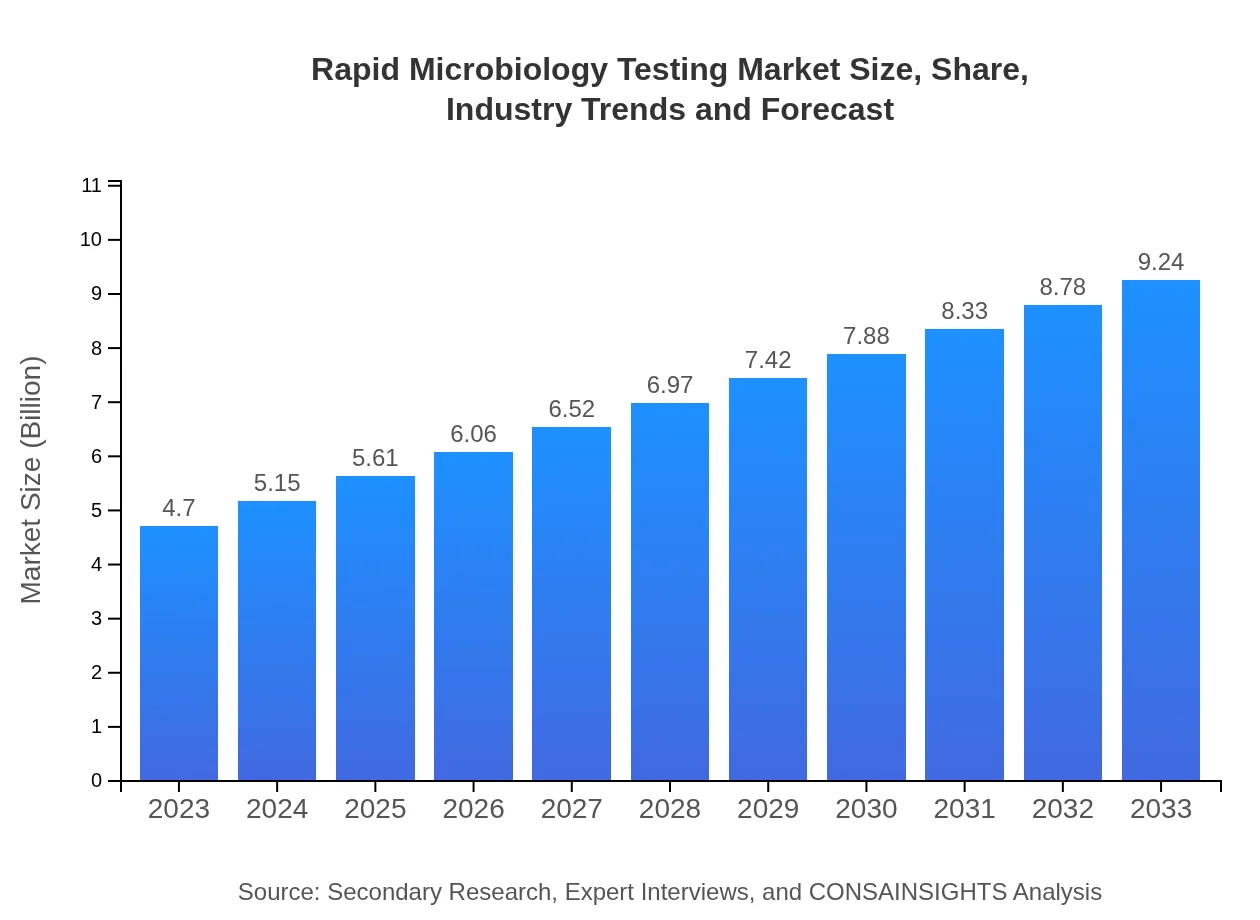
| 2023 Market Size | $4.70 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $9.24 Billion |
| Top Companies | Thermo Fisher Scientific, Roche Diagnostics, BD (Becton, Dickinson and Company), bioMérieux |
| Last Modified Date | 31 January 2026 |
Rapid Microbiology Testing Market Overview
Customize Rapid Microbiology Testing Market Report market research report
- ✔ Get in-depth analysis of Rapid Microbiology Testing market size, growth, and forecasts.
- ✔ Understand Rapid Microbiology Testing's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Rapid Microbiology Testing
What is the Market Size & CAGR of Rapid Microbiology Testing market in 2023?
Rapid Microbiology Testing Industry Analysis
Rapid Microbiology Testing Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Rapid Microbiology Testing Market Analysis Report by Region
Europe Rapid Microbiology Testing Market Report:
Europe's market stands at $1.47 billion in 2023 and is set to reach $2.90 billion by 2033, supported by stringent regulatory standards for food safety and clinical testing, as well as a growing emphasis on rapid diagnostic tests in clinical settings.Asia Pacific Rapid Microbiology Testing Market Report:
In 2023, the Asia Pacific Rapid Microbiology Testing market is valued at $0.94 billion, expected to grow to $1.85 billion by 2033. The region's growth is fueled by increasing investments in healthcare infrastructure, rising population awareness about infectious diseases, and a growing economy that enables better healthcare access.North America Rapid Microbiology Testing Market Report:
North America dominates the market with a size of $1.55 billion in 2023, anticipated to reach $3.04 billion by 2033. The expansion is driven by advanced healthcare infrastructure, high R&D investments, and a strong focus on disease prevention and control.South America Rapid Microbiology Testing Market Report:
The South American market is projected to grow from $0.21 billion in 2023 to $0.42 billion by 2033. The rise is attributed to an increase in food safety regulations and growing healthcare needs, although slower economic growth may pose challenges.Middle East & Africa Rapid Microbiology Testing Market Report:
In 2023, the Middle East and Africa market value is $0.52 billion, projected to double to $1.02 billion by 2033. Enhancement in laboratory facilities and increased funding for health initiatives are key drivers.Tell us your focus area and get a customized research report.
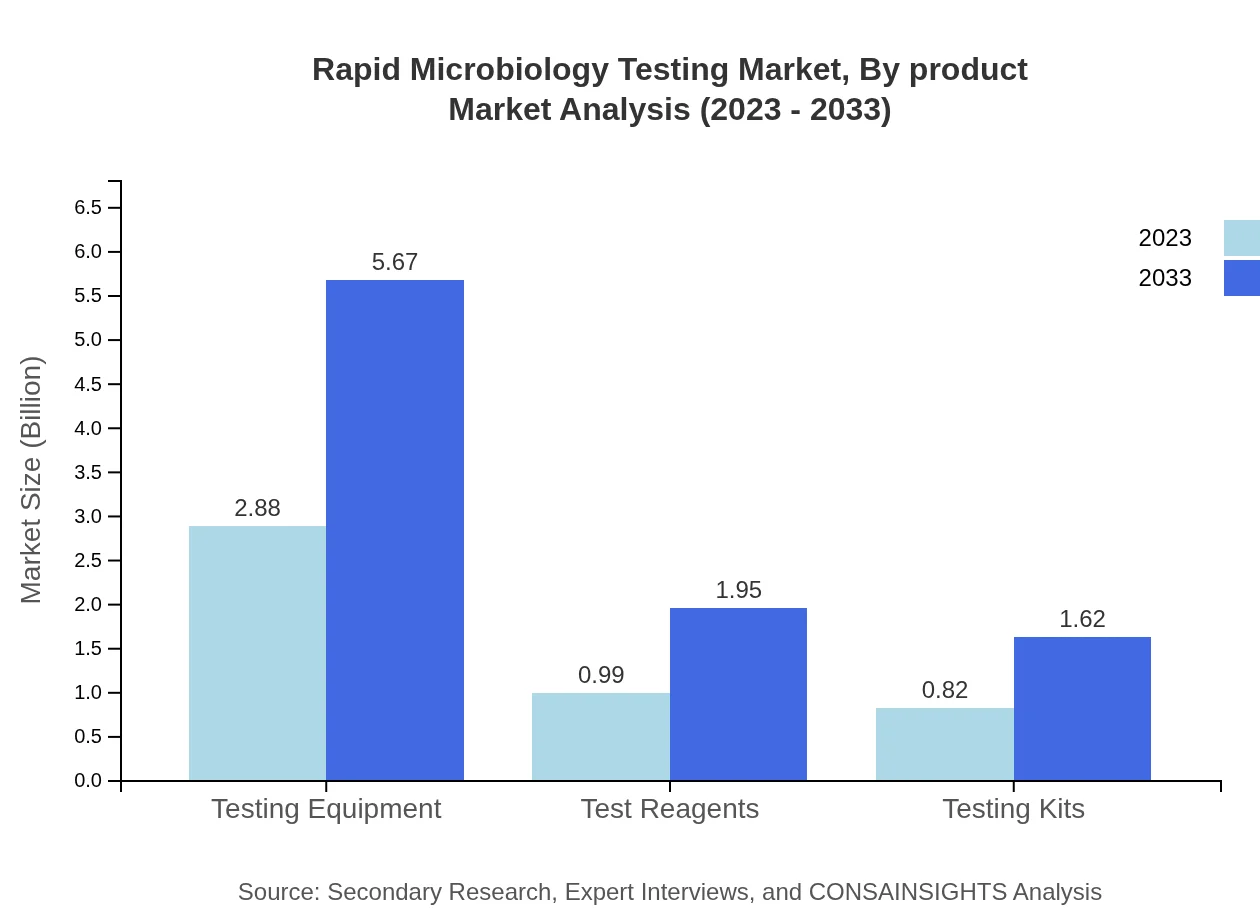
Rapid Microbiology Testing Market Analysis By Product
The Testing Equipment segment leads the market with a size of $2.88 billion in 2023, expected to rise to $5.67 billion by 2033, capturing 61.38% market share due to its essential role in rapid testing protocols. Test Reagents and Testing Kits, with sizes of $0.99 billion and $0.82 billion respectively in 2023, also contribute significantly, accounting for 21.13% and 17.49% market shares.
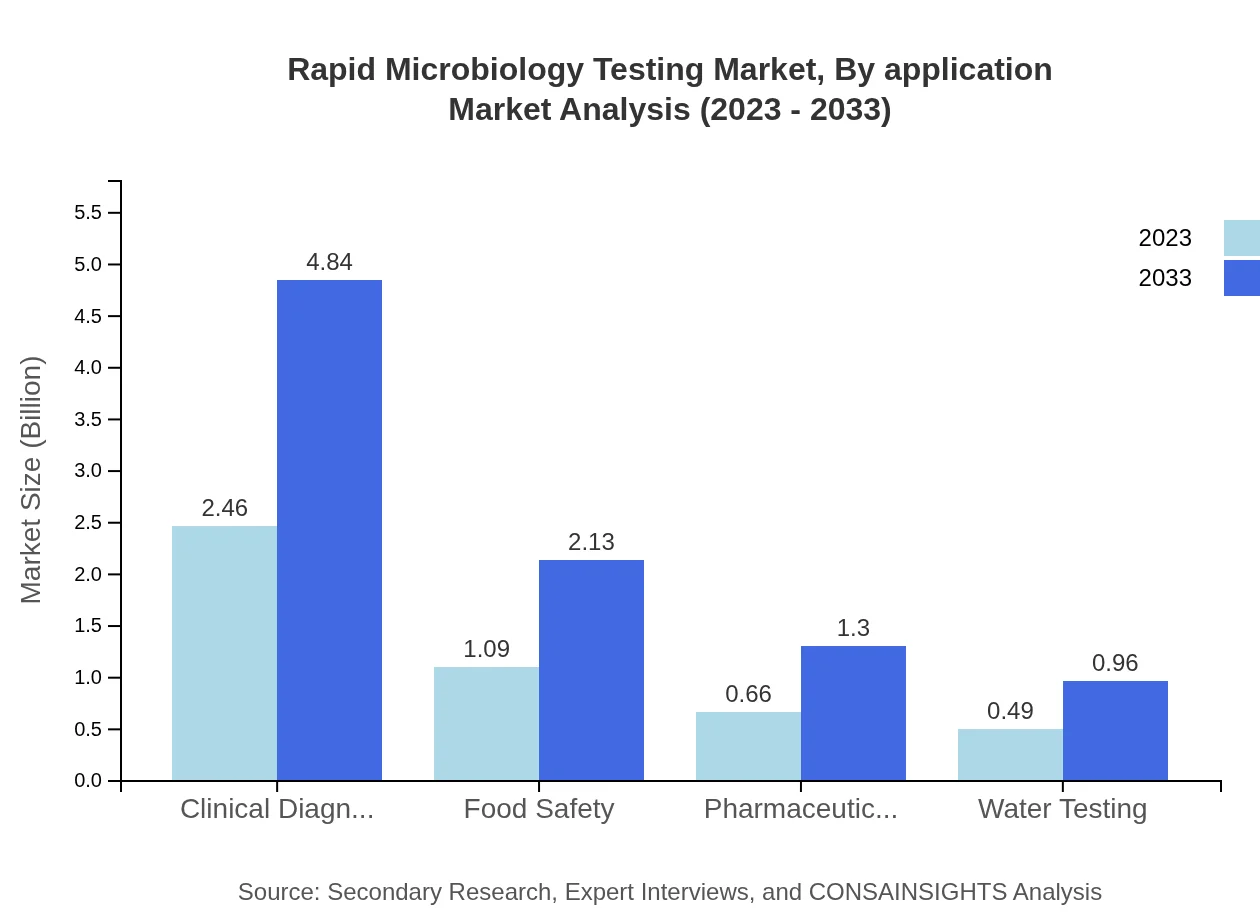
Rapid Microbiology Testing Market Analysis By Application
Clinical Diagnostics is the leading application area, generating $2.46 billion in 2023 and expected to double to $4.84 billion by 2033 with a market share of 52.41%. Food Safety and Pharmaceutical Industry applications are also critical, valued at $1.09 billion and $0.66 billion in 2023.
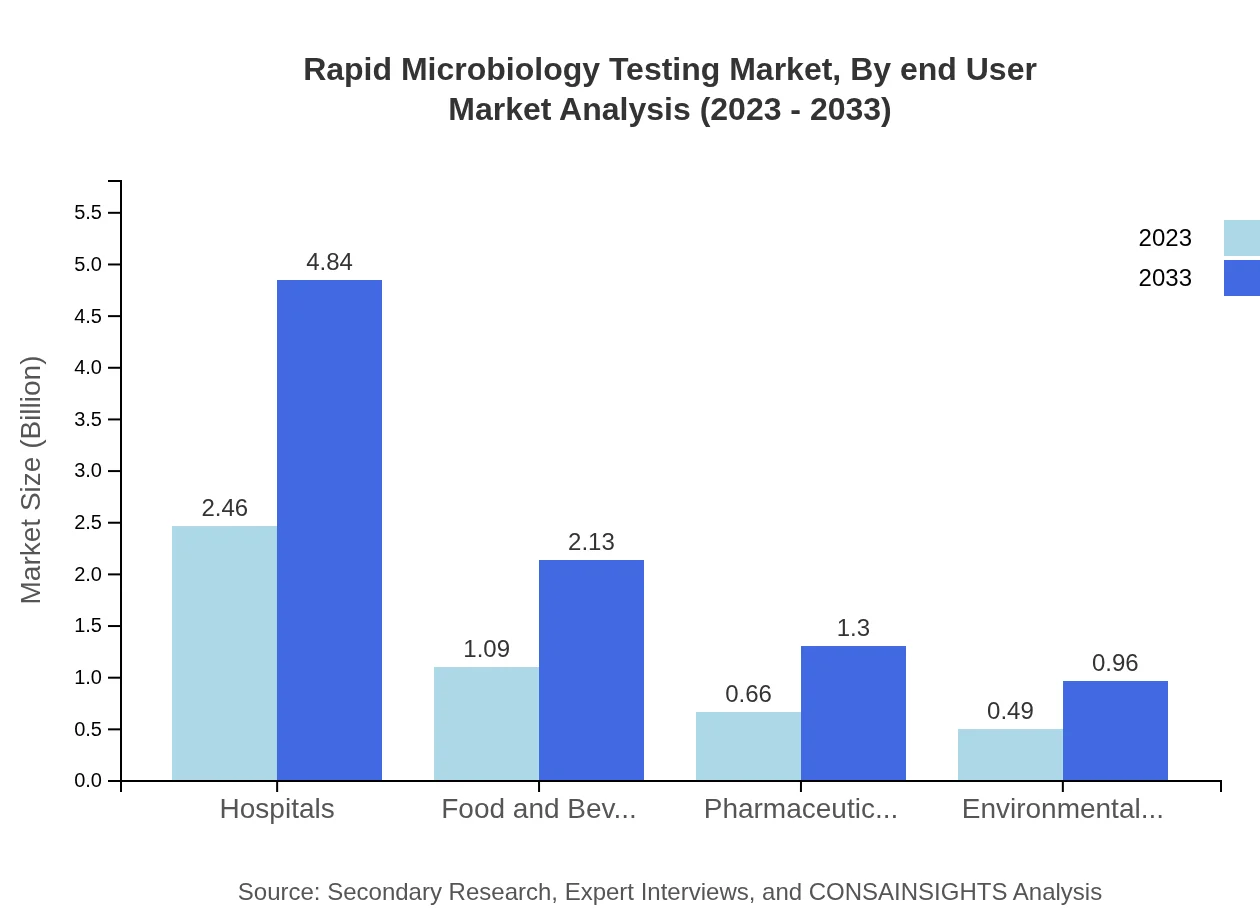
Rapid Microbiology Testing Market Analysis By End User
Hospitals dominate the end-user market with a size of $2.46 billion in 2023 and a forecast of $4.84 billion by 2033 (52.41% market share). The Food and Beverage sector follows closely behind, highlighting the critical need for safety standards in food production.
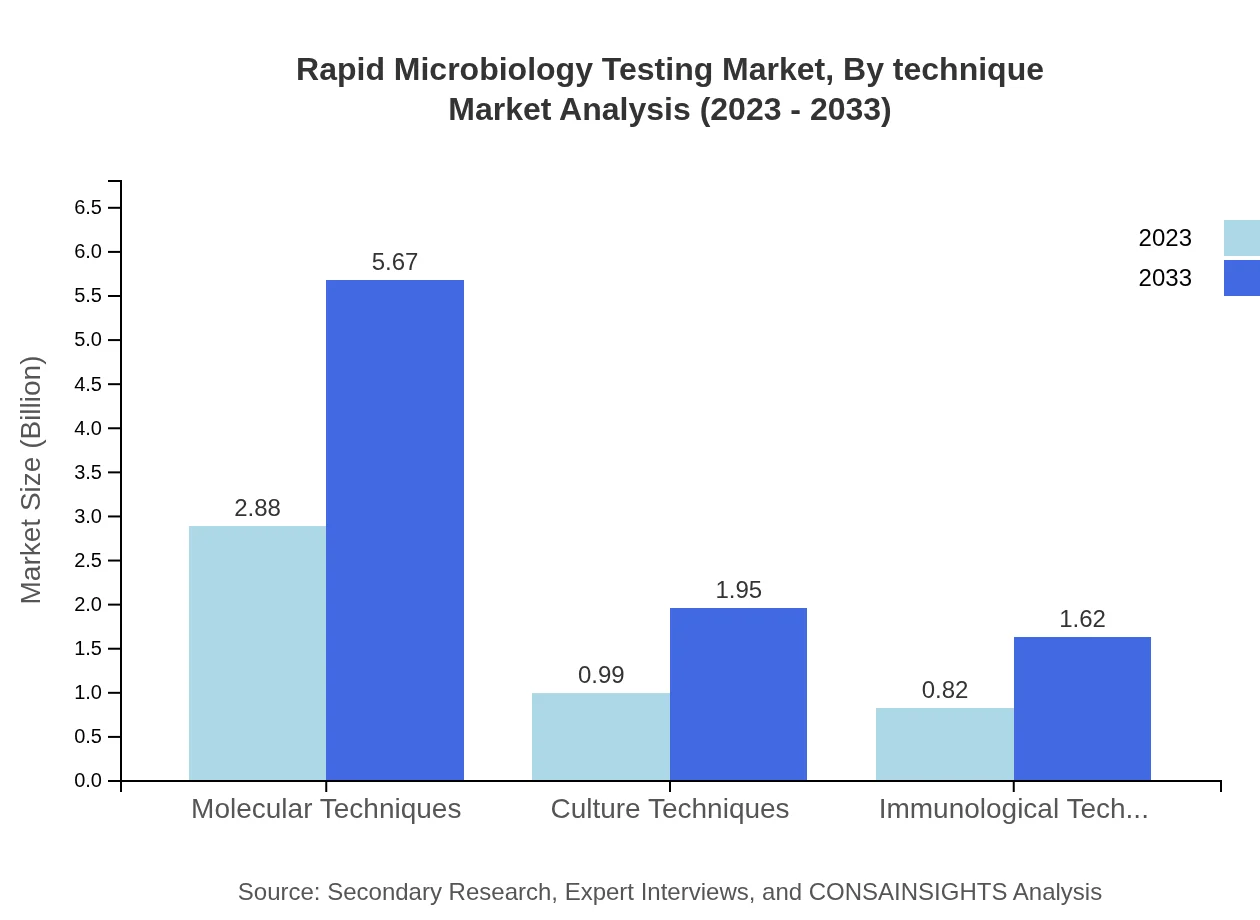
Rapid Microbiology Testing Market Analysis By Technique
Markets for Molecular Techniques and Culture Techniques are prominent, valued at $2.88 billion and $0.99 billion in 2023, respectively, emphasizing the industry's reliance on advanced techniques for effective testing.
Rapid Microbiology Testing Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Rapid Microbiology Testing Industry
Thermo Fisher Scientific:
A leading global provider of laboratory products, Thermo Fisher Scientific offers a robust portfolio of microbiology testing products, streamlining testing protocols with innovative technologies.Roche Diagnostics:
Roche Diagnostics is a major player in the healthcare industry, providing high-quality rapid testing solutions and contributing significantly to the advancement of microbial testing methodologies.BD (Becton, Dickinson and Company):
As a prominent global company in medical technology, BD develops and manufactures medical supplies and devices, playing a crucial role in microbiology testing equipment and product innovation.bioMérieux:
bioMérieux specializes in diagnostics and microbiology tests, recognized for its innovative solutions that enhance patient safety and improve diagnostic accuracy.We're grateful to work with incredible clients.









FAQs
What is the market size of rapid Microbiology Testing?
The rapid microbiology testing market is projected to reach $4.7 billion by 2033, growing at a CAGR of 6.8%. This growth is indicative of the increasing demand for quick and accurate microbiological testing across various sectors.
What are the key market players or companies in this rapid Microbiology Testing industry?
Key players in the rapid microbiology testing industry include Thermo Fisher Scientific, Becton Dickinson, and bioMérieux, among others. These companies lead with innovative products and technologies catering to laboratories and healthcare facilities.
What are the primary factors driving the growth in the rapid Microbiology Testing industry?
Factors driving growth include increased food safety concerns, rising healthcare-associated infections, and advancements in technology. These aspects fuel demand for rapid testing methods, enabling timely decision-making in clinical and environmental applications.
Which region is the fastest Growing in the rapid Microbiology Testing?
The fastest-growing region in the rapid microbiology testing market is North America, projected to grow from $1.55 billion in 2023 to $3.04 billion by 2033, driven by stringent regulations and heightened public health awareness.
Does ConsaInsights provide customized market report data for the rapid Microbiology Testing industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the rapid microbiology testing industry, ensuring clients receive insights that align with their strategic objectives.
What deliverables can I expect from this rapid Microbiology Testing market research project?
Deliverables include a comprehensive market analysis report, trends, growth forecasts, competitive landscape assessments, and tailored insights that facilitate informed decision-making and strategic planning.
What are the market trends of rapid Microbiology Testing?
Current trends include increasing adoption of automation and digitalization in testing, a shift towards molecular techniques, and heightened investments in food safety and healthcare diagnostics, underscoring a proactive approach to microbiological threats.