Rapid Plasma Reagin Test Market Report
Published Date: 31 January 2026 | Report Code: rapid-plasma-reagin-test
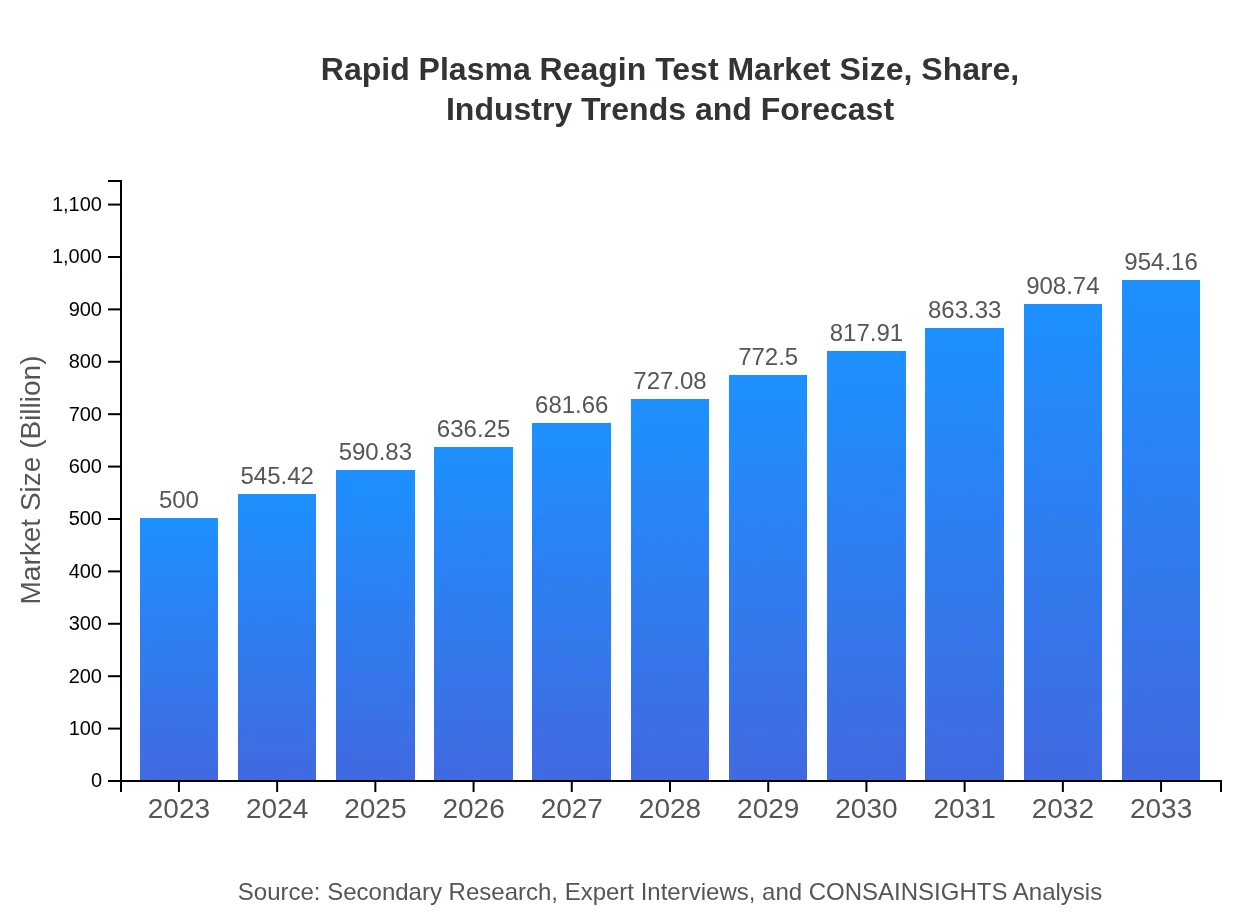
Rapid Plasma Reagin Test Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Rapid Plasma Reagin Test market, covering market size, CAGR, industry analysis, segmentation, regional insights, technology trends, and forecasts from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $500.00 Million |
| CAGR (2023-2033) | 6.5% |
| 2033 Market Size | $954.16 Million |
| Top Companies | Abbott Laboratories, Roche Diagnostics, BD (Becton, Dickinson and Company), Thermo Fisher Scientific |
| Last Modified Date | 31 January 2026 |
Rapid Plasma Reagin Test Market Overview
Customize Rapid Plasma Reagin Test Market Report market research report
- ✔ Get in-depth analysis of Rapid Plasma Reagin Test market size, growth, and forecasts.
- ✔ Understand Rapid Plasma Reagin Test's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Rapid Plasma Reagin Test
What is the Market Size & CAGR of Rapid Plasma Reagin Test market in 2023 and 2033?
Rapid Plasma Reagin Test Industry Analysis
Rapid Plasma Reagin Test Market Segmentation and Scope
Tell us your focus area and get a customized research report.
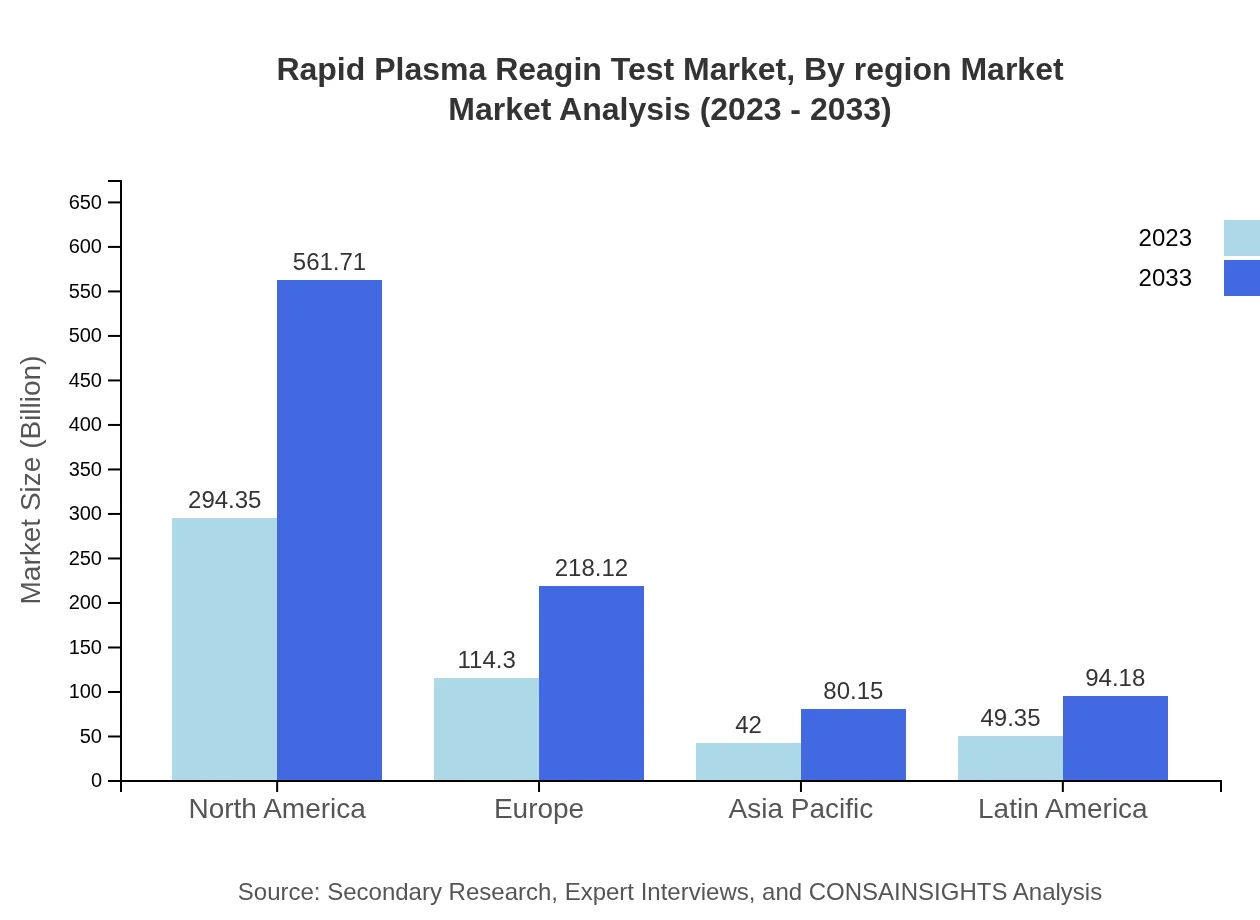
Rapid Plasma Reagin Test Market Analysis Report by Region
Europe Rapid Plasma Reagin Test Market Report:
The European market for the Rapid Plasma Reagin Test is expected to experience growth from $136.80 million in 2023 to $261.06 million by 2033. Increased government funding for STI prevention and testing initiatives, alongside a rising number of reported syphilis cases, contributes to this growth.Asia Pacific Rapid Plasma Reagin Test Market Report:
In the Asia Pacific region, the Rapid Plasma Reagin Test market is expanding due to rising healthcare expenditure and government initiatives to combat infectious diseases. The market is expected to grow from $108.85 million in 2023 to $207.72 million by 2033, driven by increasing awareness and accessibility of testing services.North America Rapid Plasma Reagin Test Market Report:
North America remains a key market for the Rapid Plasma Reagin Test, predominantly due to the high incidence of syphilis and the existence of advanced healthcare facilities. The market is forecasted to grow from $167.60 million in 2023 to $319.83 million by 2033, supported by technological advancements in diagnostic tools.South America Rapid Plasma Reagin Test Market Report:
South America presents significant growth potential for the Rapid Plasma Reagin Test market, with a market size projected to increase from $49.15 million in 2023 to $93.79 million by 2033. Efforts in public health campaigns and improved healthcare infrastructure are anticipated to drive demand in this region.Middle East & Africa Rapid Plasma Reagin Test Market Report:
The Middle East and Africa market is projected to transition from $37.60 million in 2023 to $71.75 million by 2033, driven by growing public awareness of STIs and increased governmental and NGO efforts in health education and testing accessibility.Tell us your focus area and get a customized research report.
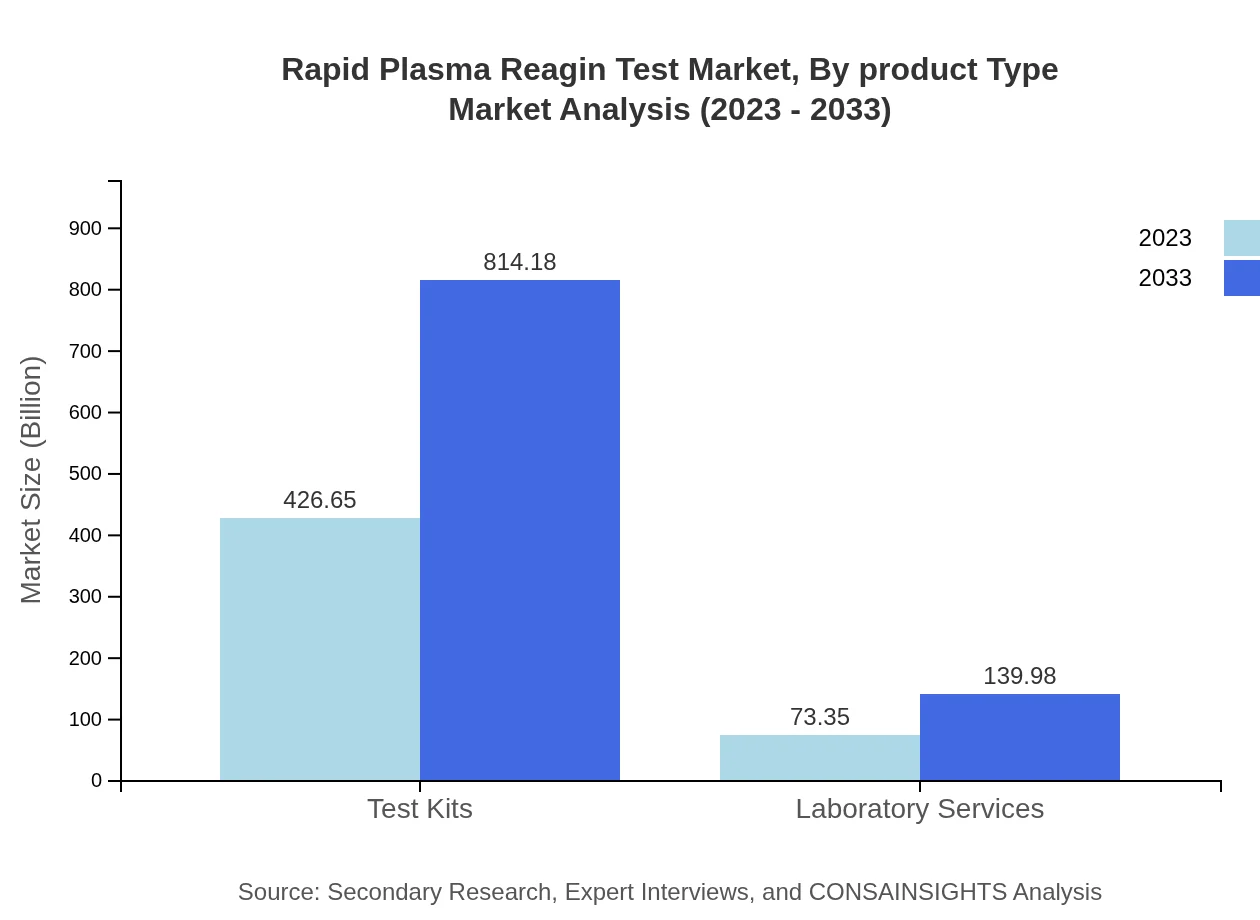
Rapid Plasma Reagin Test Market Analysis By Product Type
The market for Rapid Plasma Reagin Test by product type includes test kits and laboratory services. Test kits dominate the market due to their convenience and rapid results. In 2023, the market size for test kits is $426.65 million, expected to grow to $814.18 million by 2033. Laboratory services, while growing, hold a smaller share at $73.35 million in 2023, with an increase to $139.98 million by 2033.
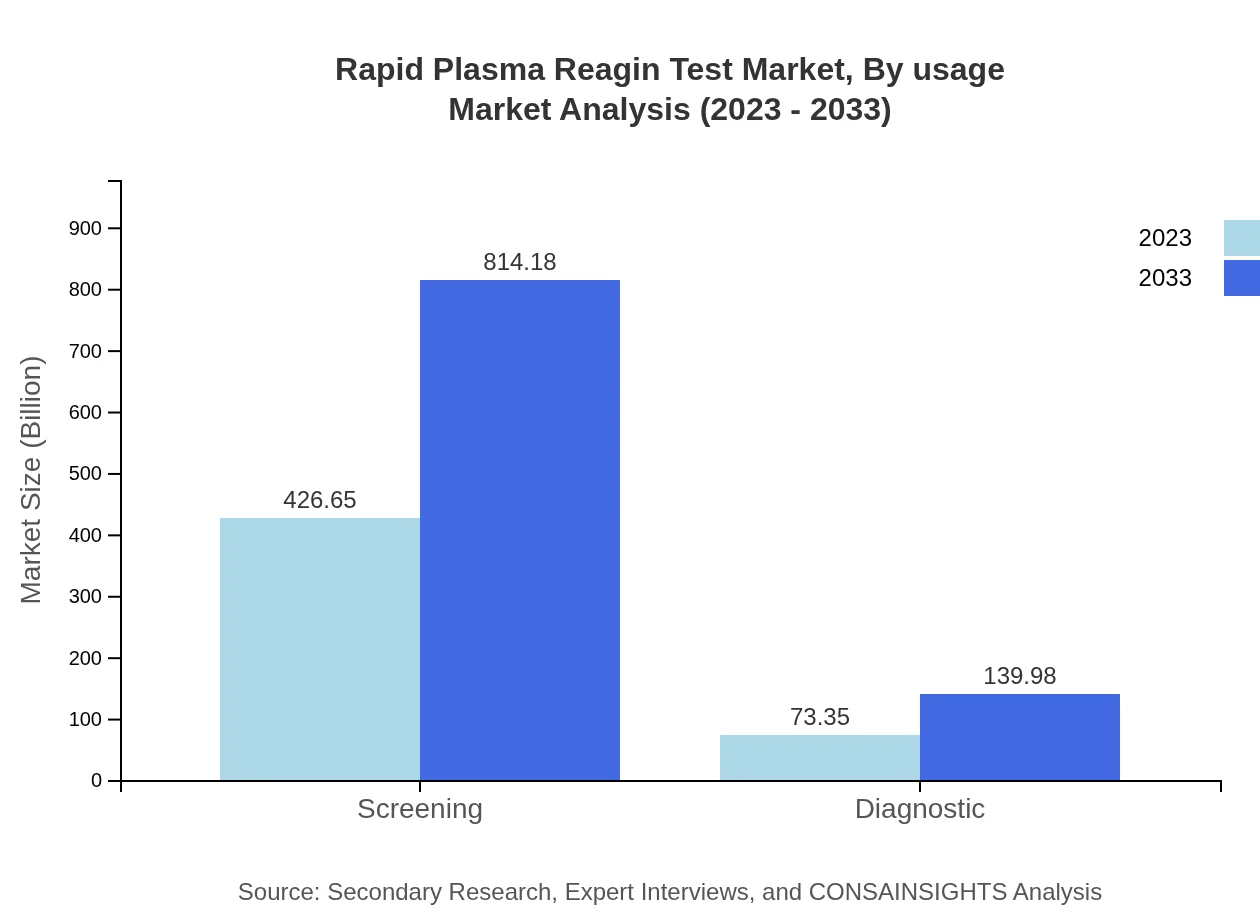
Rapid Plasma Reagin Test Market Analysis By Usage
In terms of usage, the market splits into screening and diagnostic segments. The screening market leads with $426.65 million in 2023, growing to $814.18 million by 2033. The diagnostic segment is smaller, starting at $73.35 million in 2023 and reaching $139.98 million by 2033, reflecting the market's emphasis on preventative screenings.
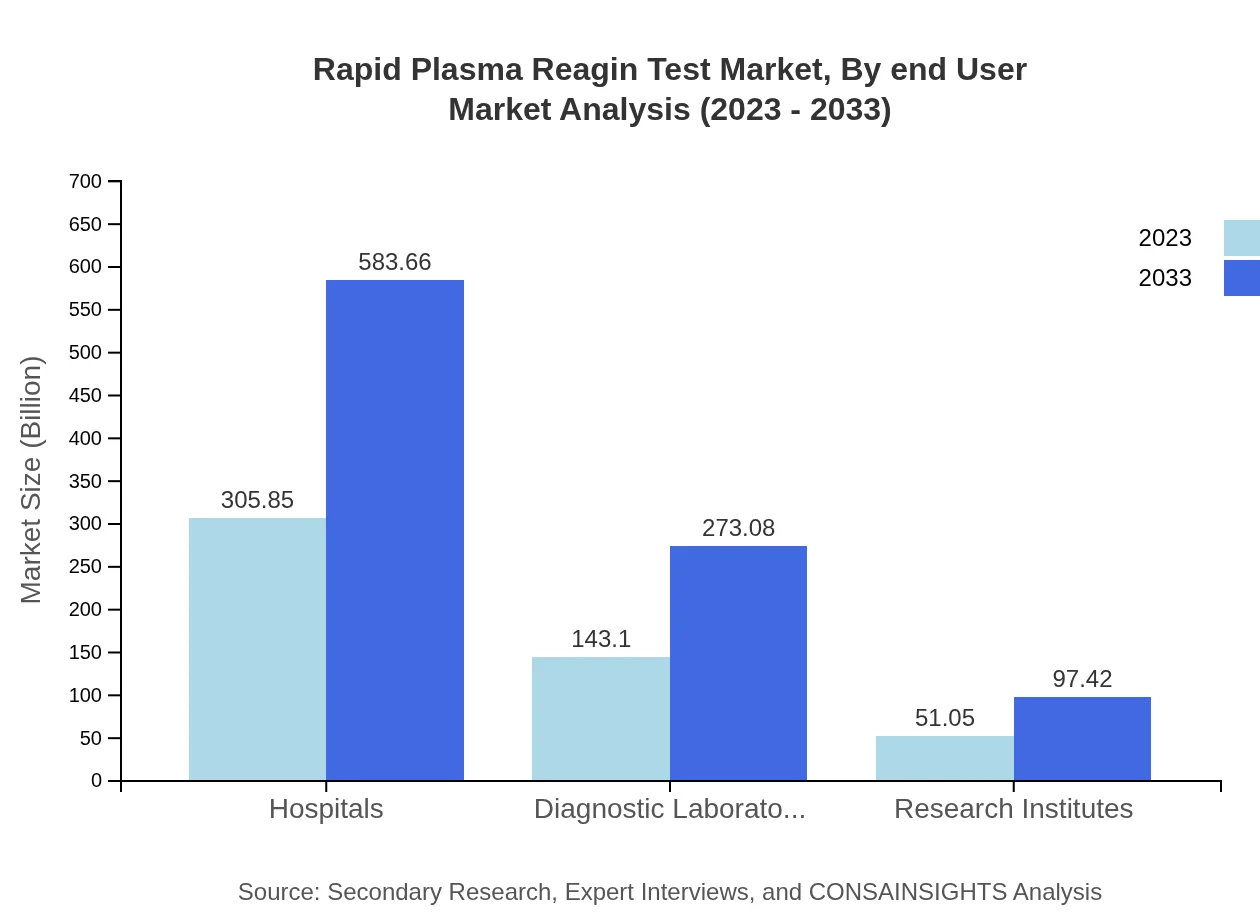
Rapid Plasma Reagin Test Market Analysis By End User
End-user segmentation shows hospitals leading the market with $305.85 million in 2023, projected to reach $583.66 million by 2033. Diagnostic laboratories and research institutes follow, with sizes of $143.10 million and $51.05 million in 2023, increasing to $273.08 million and $97.42 million respectively.
Rapid Plasma Reagin Test Market Analysis By Region Market
Regional analysis indicates North America as a primary market with significant growth potential. Europe, Asia Pacific, and South America also exhibit expanding markets, propelled by increasing awareness and supportive policies regarding STIs. The Middle East and Africa is gradually enhancing its market share through focused health initiatives.
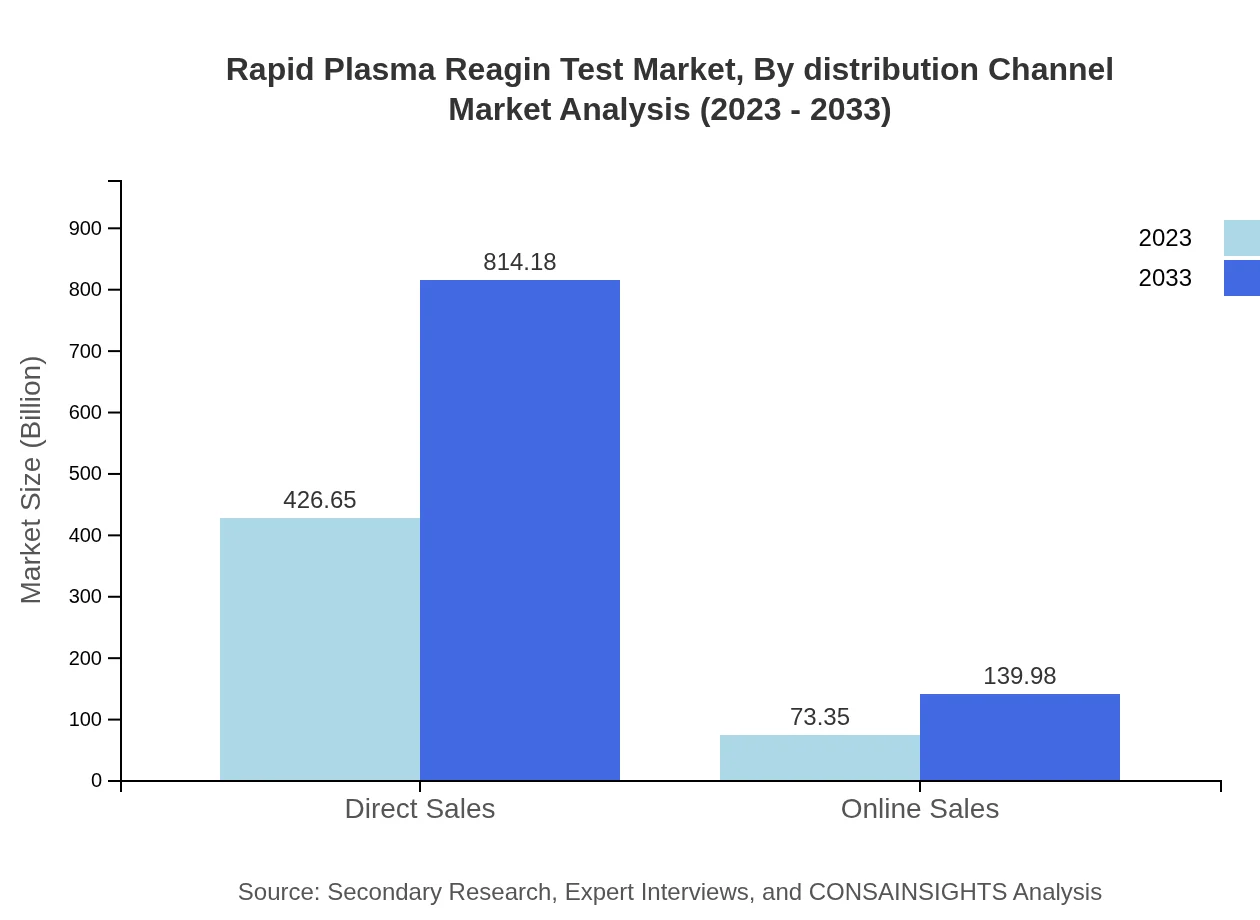
Rapid Plasma Reagin Test Market Analysis By Distribution Channel
Distribution channels for the Rapid Plasma Reagin Test include direct sales and online sales. Direct sales command the market with $426.65 million in 2023, forecasting an increase to $814.18 million by 2033. Online sales present a growing segment with initial revenues of $73.35 million, expected to rise to $139.98 million.
Rapid Plasma Reagin Test Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Rapid Plasma Reagin Test Industry
Abbott Laboratories:
Abbott is a major global player in diagnostics, providing innovative testing solutions, including rapid plasma reagin tests, indicative of its commitment to quality healthcare.Roche Diagnostics:
Roche specializes in cutting-edge diagnostics, offering comprehensive RPR testing solutions that are widely used across healthcare facilities.BD (Becton, Dickinson and Company):
BD is known for its advanced medical technologies and diagnostics, including RPR testing kits that facilitate efficient syphilis screening.Thermo Fisher Scientific:
Thermo Fisher provides an extensive range of testing services, including RPR tests, noted for their reliability and precision in disease diagnosis.We're grateful to work with incredible clients.









FAQs
What is the market size of Rapid Plasma Reagin Test?
The global market size for the Rapid Plasma Reagin Test is estimated at $500 million in 2023, with a projected CAGR of 6.5% from 2023 to 2033, indicating robust growth in the industry as demand rises.
What are the key market players or companies in the Rapid Plasma Reagin Test industry?
Key players in the Rapid Plasma Reagin Test industry include major pharmaceutical companies and medical laboratories that specialize in diagnostic test kits and laboratory services, contributing significantly to the market with innovative products and competitive pricing.
What are the primary factors driving the growth in the Rapid Plasma Reagin Test industry?
Growth in the Rapid Plasma Reagin Test market is driven by increasing syphilis infection rates, greater awareness of sexually transmitted diseases, technological advancements in testing methods, and rising demand for rapid and accurate diagnostic tests.
Which region is the fastest Growing in the Rapid Plasma Reagin Test?
North America is the fastest-growing region in the Rapid Plasma Reagin Test market, projected to expand from $167.60 million in 2023 to $319.83 million by 2033, highlighting significant market potential and investment opportunities.
Does ConsaInsights provide customized market report data for the Rapid Plasma Reagin Test industry?
Yes, ConsaInsights offers customized market report data tailored to your specific needs in the Rapid Plasma Reagin Test industry, allowing clients to access targeted insights and analyses for strategic decision-making.
What deliverables can I expect from this Rapid Plasma Reagin Test market research project?
Deliverables from the Rapid Plasma Reagin Test market research project include comprehensive market analysis reports, competitor profiling, trend evaluations, forecast models, and recommendations for key stakeholders in the industry.
What are the market trends of Rapid Plasma Reagin Test?
Current trends in the Rapid Plasma Reagin Test market include an increase in demand for home testing kits, advancements in technology for faster results, focus on patient-centric solutions, and expansion of testing services in various healthcare settings.