Rare Hemophilia Factors Market Report
Published Date: 31 January 2026 | Report Code: rare-hemophilia-factors
Rare Hemophilia Factors Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Rare Hemophilia Factors market, presenting insights on market trends, sizes, and forecasts from 2023 to 2033. Key segments are explored, along with regional breakdowns and competitive analyses of the leading companies in the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
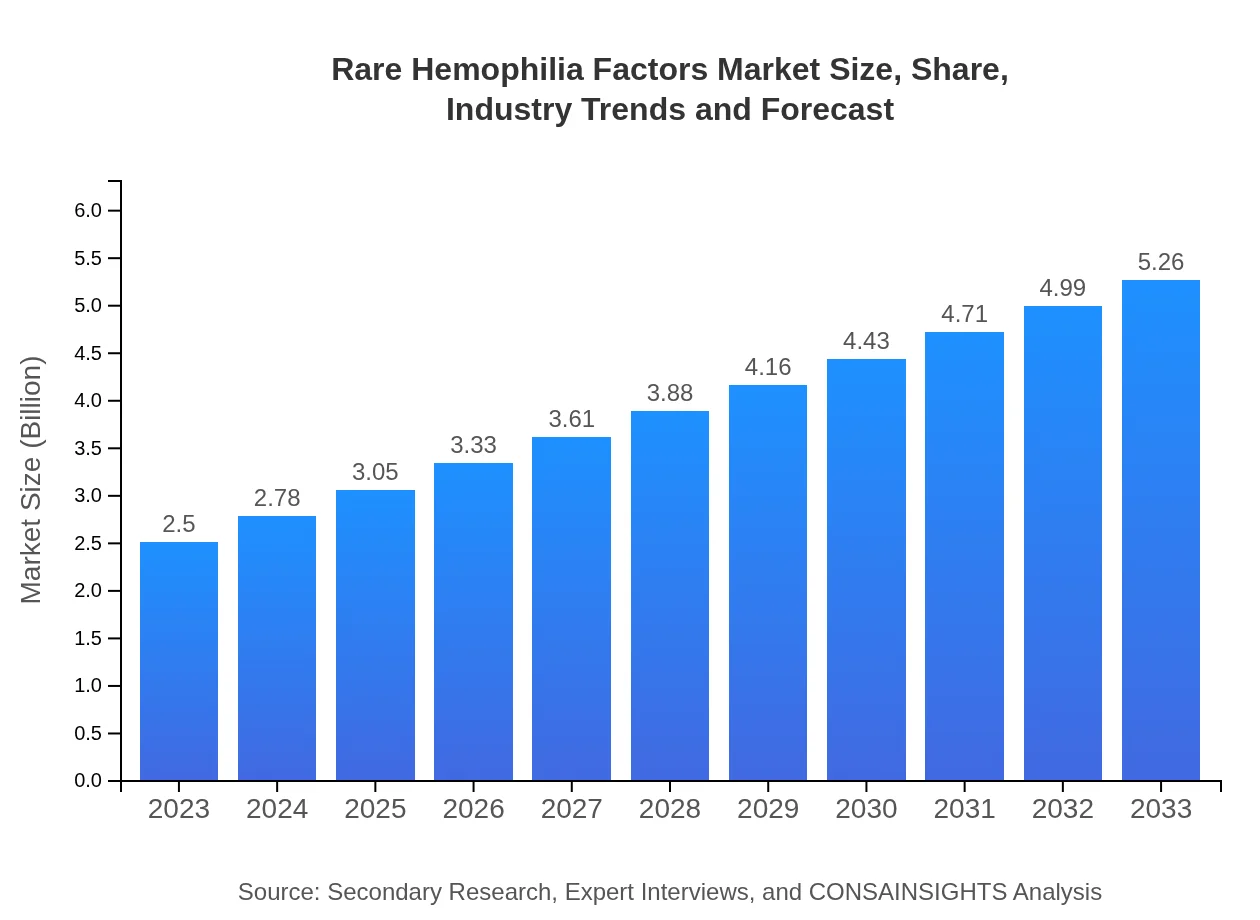
| 2023 Market Size | $2.50 Billion |
| CAGR (2023-2033) | 7.5% |
| 2033 Market Size | $5.26 Billion |
| Top Companies | Bayer AG, CSL Behring, Shire (now part of Takeda Pharmaceutical Company) |
| Last Modified Date | 31 January 2026 |
Rare Hemophilia Factors Market Overview
Customize Rare Hemophilia Factors Market Report market research report
- ✔ Get in-depth analysis of Rare Hemophilia Factors market size, growth, and forecasts.
- ✔ Understand Rare Hemophilia Factors's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Rare Hemophilia Factors
What is the Market Size & CAGR of Rare Hemophilia Factors market in 2023?
Rare Hemophilia Factors Industry Analysis
Rare Hemophilia Factors Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Rare Hemophilia Factors Market Analysis Report by Region
Europe Rare Hemophilia Factors Market Report:
Europe, with its comprehensive healthcare policies, is projected to witness growth from $0.67 billion in 2023 to $1.40 billion by 2033. Countries with strong healthcare frameworks, such as Germany, France, and the UK, are focused on enhancing treatment options for hemophilia, driving market growth through innovation.Asia Pacific Rare Hemophilia Factors Market Report:
The Asia Pacific region is experiencing significant growth in the Rare Hemophilia Factors market, with a projected market value of $1.03 billion by 2033, up from $0.49 billion in 2023. Countries like China and India are increasing their healthcare investments to improve access to rare disease treatments, thereby expanding patient outreach and market potential.North America Rare Hemophilia Factors Market Report:
The North American market, led by the United States, is expected to grow from $0.86 billion in 2023 to an impressive $1.82 billion by 2033. This significant growth is underpinned by advanced healthcare systems, high treatment adoption rates, and the presence of major pharmaceutical companies innovating in rare disease therapies.South America Rare Hemophilia Factors Market Report:
In South America, the market is expanding steadily with an anticipated increase from $0.18 billion in 2023 to $0.37 billion by 2033. Stimulated by improved healthcare infrastructure and awareness of hemophilia and related disorders, growth in this region is reinforced by partnerships between governments and healthcare organizations addressing specialty medications.Middle East & Africa Rare Hemophilia Factors Market Report:
The Middle East and Africa region is seeing growth from $0.31 billion in 2023 to $0.64 billion by 2033. Investment in healthcare reforms and increased governmental support for rare disease management are crucial factors driving this growth, along with the increasing prevalence of hemophilia.Tell us your focus area and get a customized research report.
Rare Hemophilia Factors Market Analysis By Factor
Factors designated for hemophilia treatment exhibit varying growth rates. Factor I's market size is anticipated to grow from $1.27 billion in 2023 to $2.68 billion by 2033. Factor II and Factor VII are also important, with values of $0.60 billion and $0.37 billion in 2023, respectively, and are expected to reach $1.27 billion and $0.77 billion in 2033.
Rare Hemophilia Factors Market Analysis By Therapy Type
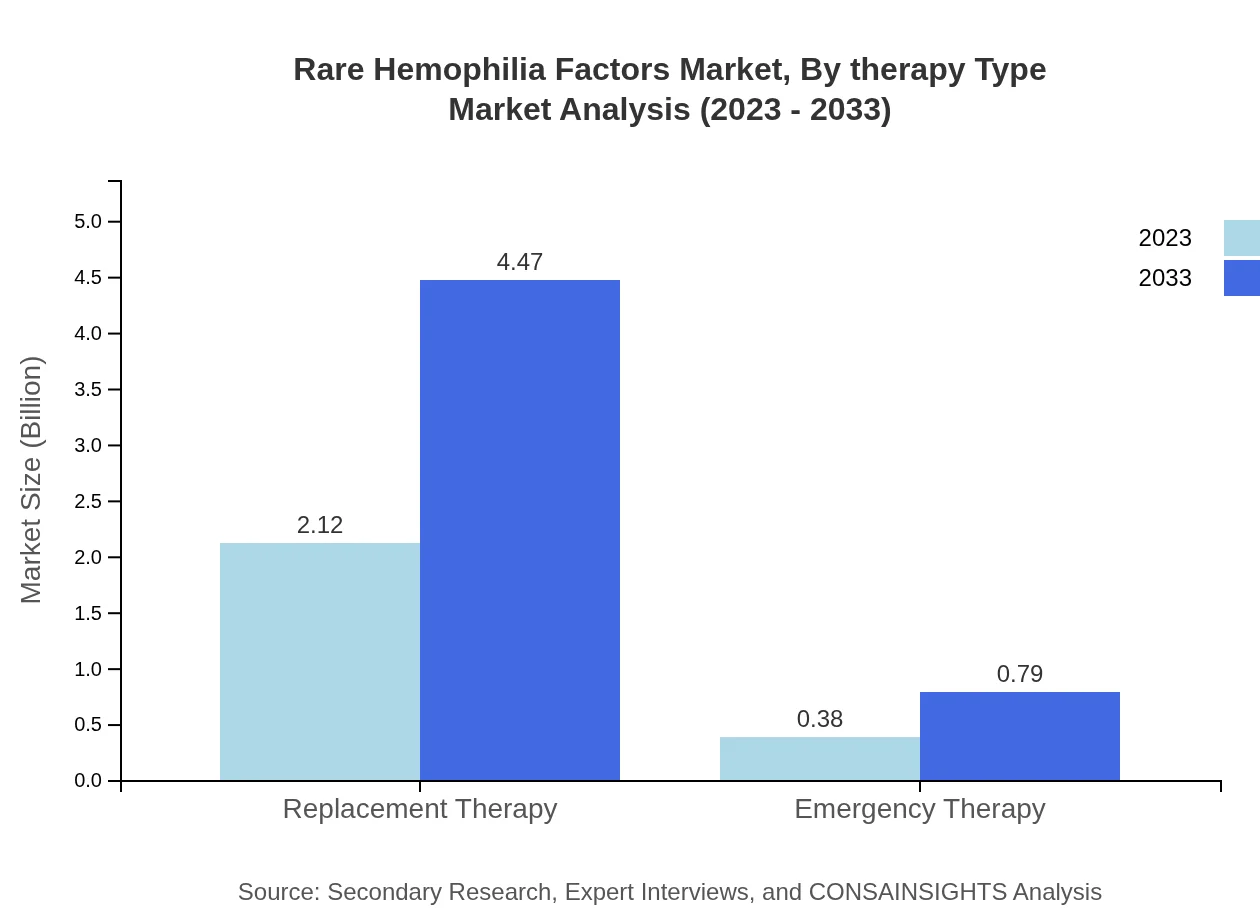
The therapy types include replacement and emergency therapies. Replacement Therapy has a dominant market share of 84.9% in 2023 and $2.12 billion in market size, growing to $4.47 billion by 2033. Emergency Therapy, while smaller, is also expected to double from $0.38 billion to $0.79 billion during the same period.
Rare Hemophilia Factors Market Analysis By Application
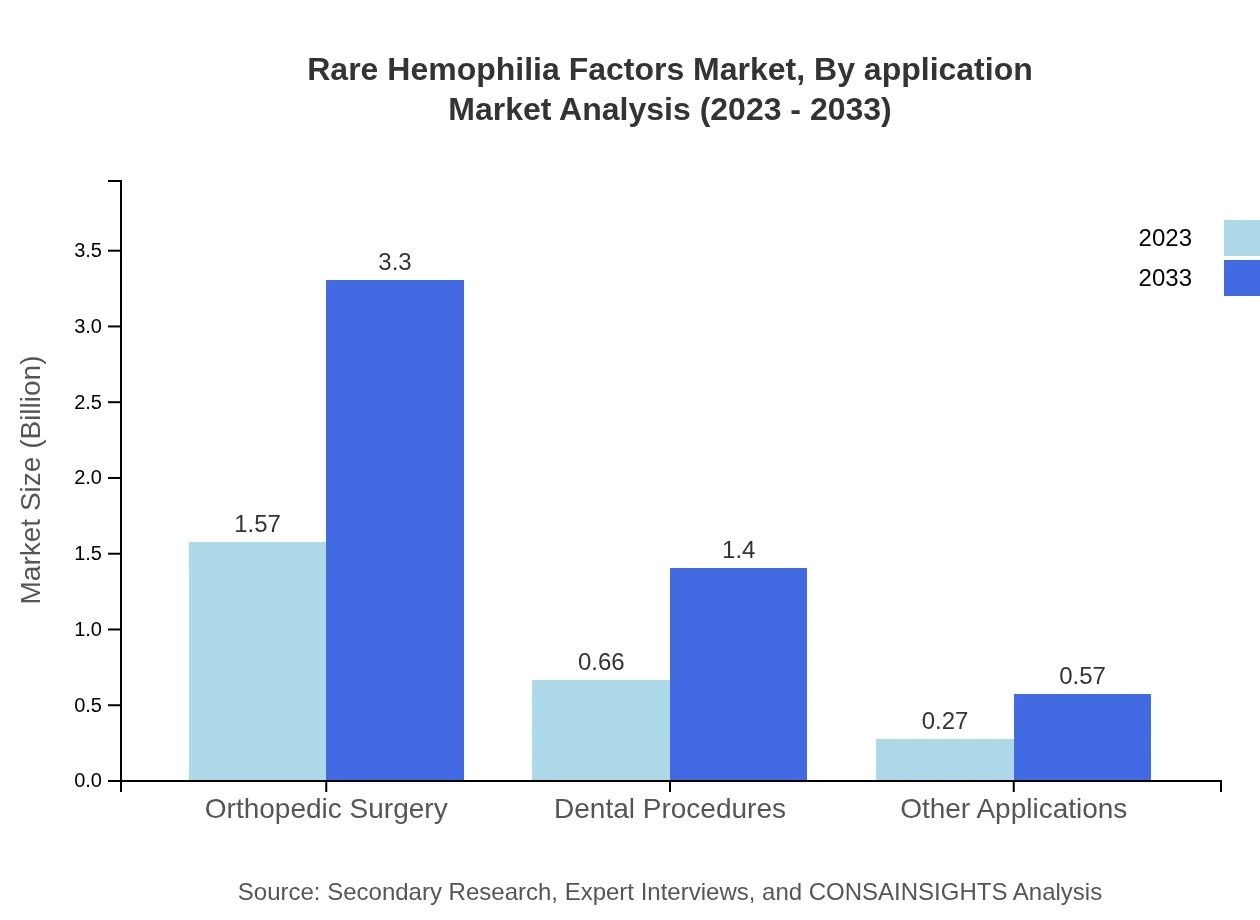
Key applications consist of hospitals, clinics, and pharmacies. Hospitals and Clinics hold considerable market shares, with expected growth from $1.57 billion in 2023 to $3.30 billion by 2033 for hospitals, while clinics are projected to climb from $0.66 billion to $1.40 billion.
Rare Hemophilia Factors Market Analysis By Distribution Channel
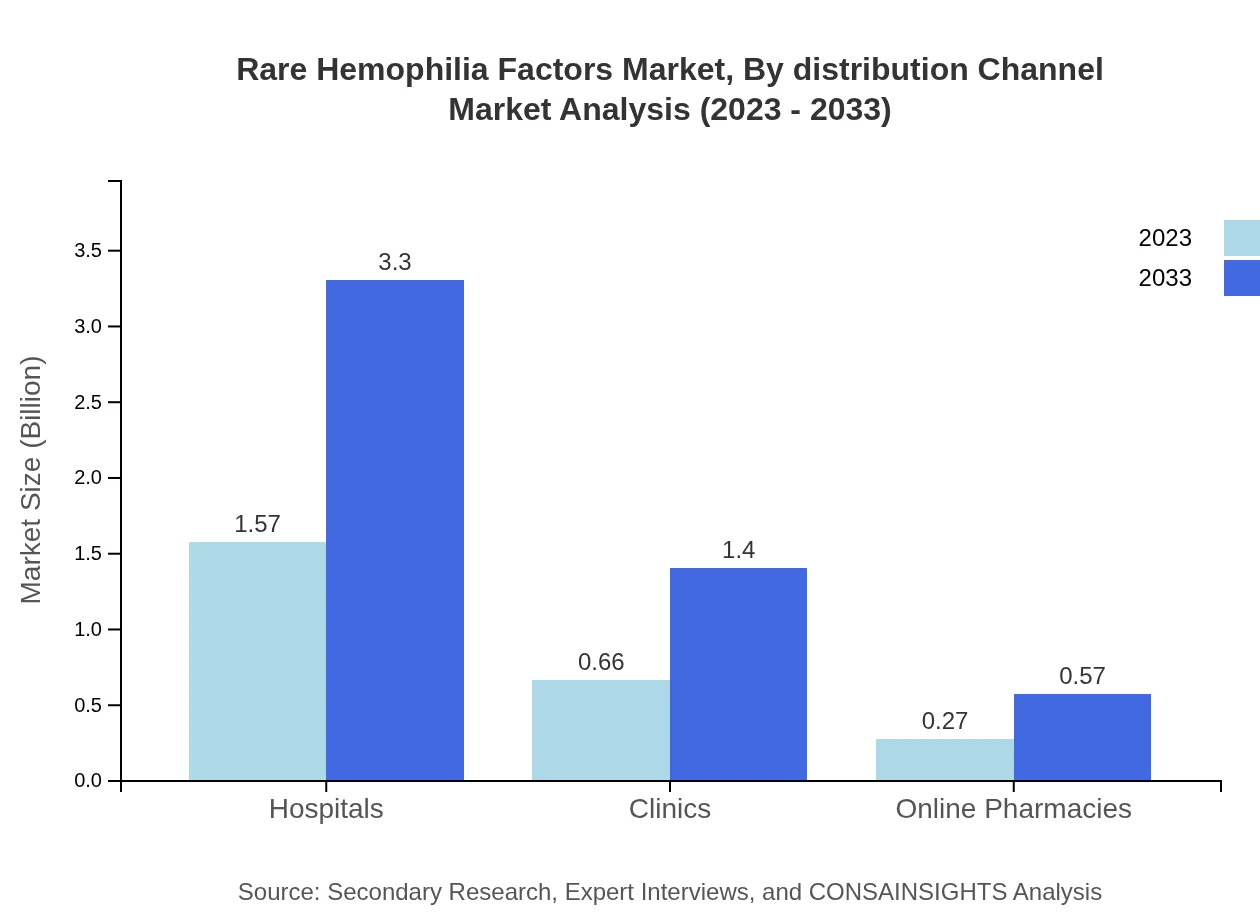
Distribution channels primarily include hospitals, clinics, online pharmacies, and home care settings. Hospital-based distribution retains a 62.64% share, reflecting its importance as patients frequently receive care in these settings. Home care settings are escalating in relevance, moving from $0.66 billion in 2023 to $1.40 billion by 2033.
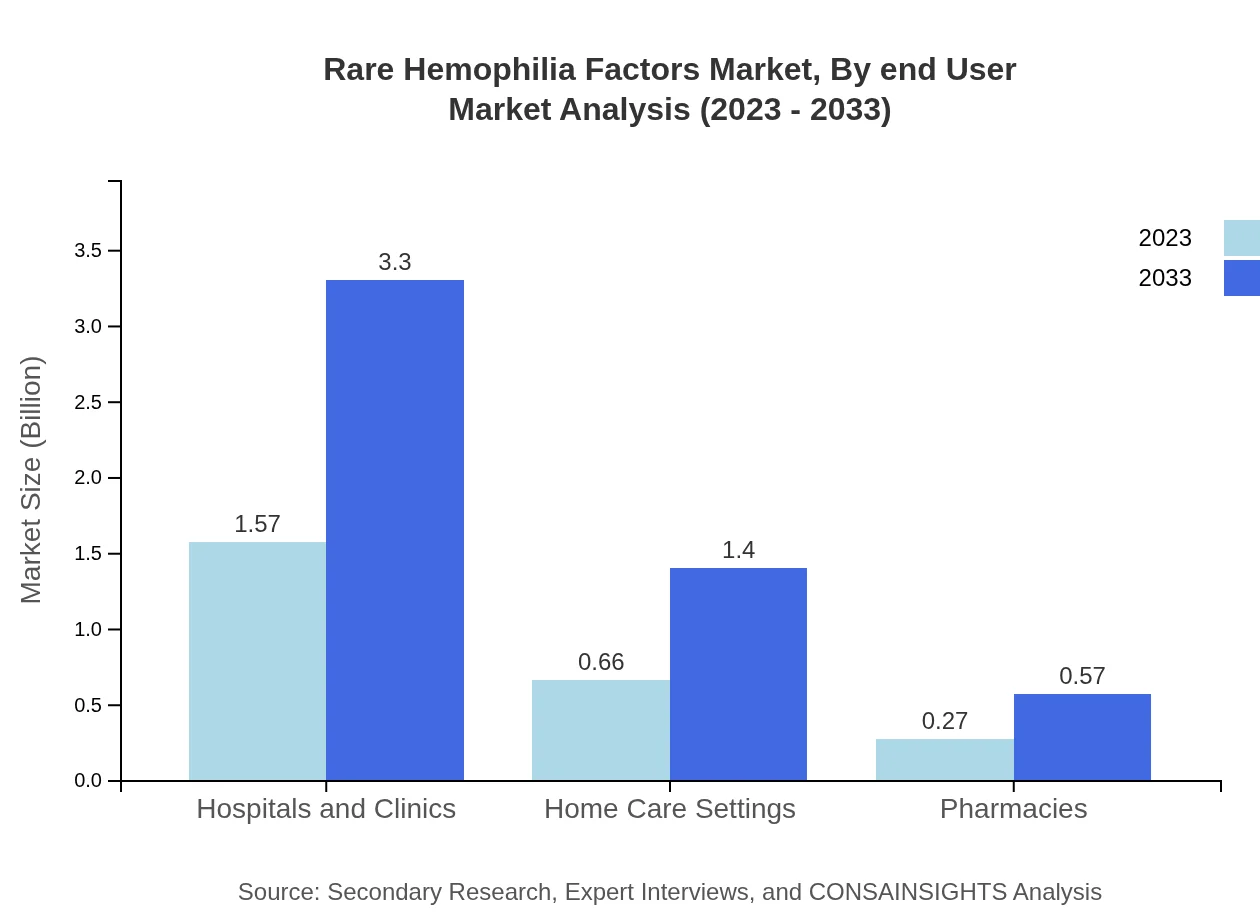
Rare Hemophilia Factors Market Analysis By End User
End-user segmentation outlines the major consumers of Rare Hemophilia Factors products. Hospitals and clinics, with their significant market share, are the largest end-users, while home care settings are seeing growing adoption as awareness about self-management of therapy increases, expected to reach a market value of $1.40 billion by 2033.
Rare Hemophilia Factors Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Rare Hemophilia Factors Industry
Bayer AG:
Bayer AG is a leading global pharmaceutical and life sciences company known for its innovative therapies including Kovaltry, a factor VIII treatment for hemophilia A, contributing significantly to market growth.CSL Behring:
CSL Behring is a global leader in biotherapy, delivering life-saving therapies for people with bleeding disorders through products like Berinert and Corifact.Shire (now part of Takeda Pharmaceutical Company):
Shire has a strong portfolio of hemophilia products, including Adynovate for hemophilia A, emphasizing patient access and innovative therapy solutions.We're grateful to work with incredible clients.









FAQs
What is the market size of rare Hemophilia Factors?
The rare hemophilia factors market is valued at approximately $2.5 billion in 2023, with a compound annual growth rate (CAGR) of 7.5% expected through 2033. This growth can be attributed to increased demand for treatment options.
What are the key market players or companies in this industry?
Key players in the rare hemophilia factors market include pharmaceutical companies specializing in blood disorders. These typically involve biotechnology firms and multinational corporations, ensuring a significant presence and competitive landscape.
What are the primary factors driving the growth in the rare Hemophilia Factors industry?
Growth is primarily driven by rising incidences of hemophilia, increased awareness, technological advancements in therapy, and improved healthcare access globally, leading to greater demand for specialized treatment solutions.
Which region is the fastest Growing in the rare Hemophilia Factors market?
The Asia Pacific region shows the fastest growth, with a market size expected to grow from $0.49 billion in 2023 to $1.03 billion by 2033, driven by healthcare improvements and rising population awareness.
Does ConsaInsights provide customized market report data for the rare Hemophilia Factors industry?
Yes, ConsaInsights offers customized market report data, allowing tailored insights and analyses for clients in the rare hemophilia factors industry, meeting specific needs and requirements.
What deliverables can I expect from this rare Hemophilia Factors market research project?
Deliverables include comprehensive market reports, trend analysis, regional evaluations, segment insights, and detailed competitive landscapes tailored to your strategic needs in the industry.
What are the market trends of rare Hemophilia Factors?
Market trends indicate a shift towards home care settings and innovative treatment modalities, with segments like Replacement Therapy leading in market share, reflecting advancements in patient care and treatment solutions.