Rare Neurological Disease Treatment Market Report
Published Date: 31 January 2026 | Report Code: rare-neurological-disease-treatment
Rare Neurological Disease Treatment Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Rare Neurological Disease Treatment market, covering market size, segmentation, growth trends, and forecasts from 2023 to 2033. Insights into regional performance and key players in the industry are also highlighted, offering valuable data for stakeholders.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
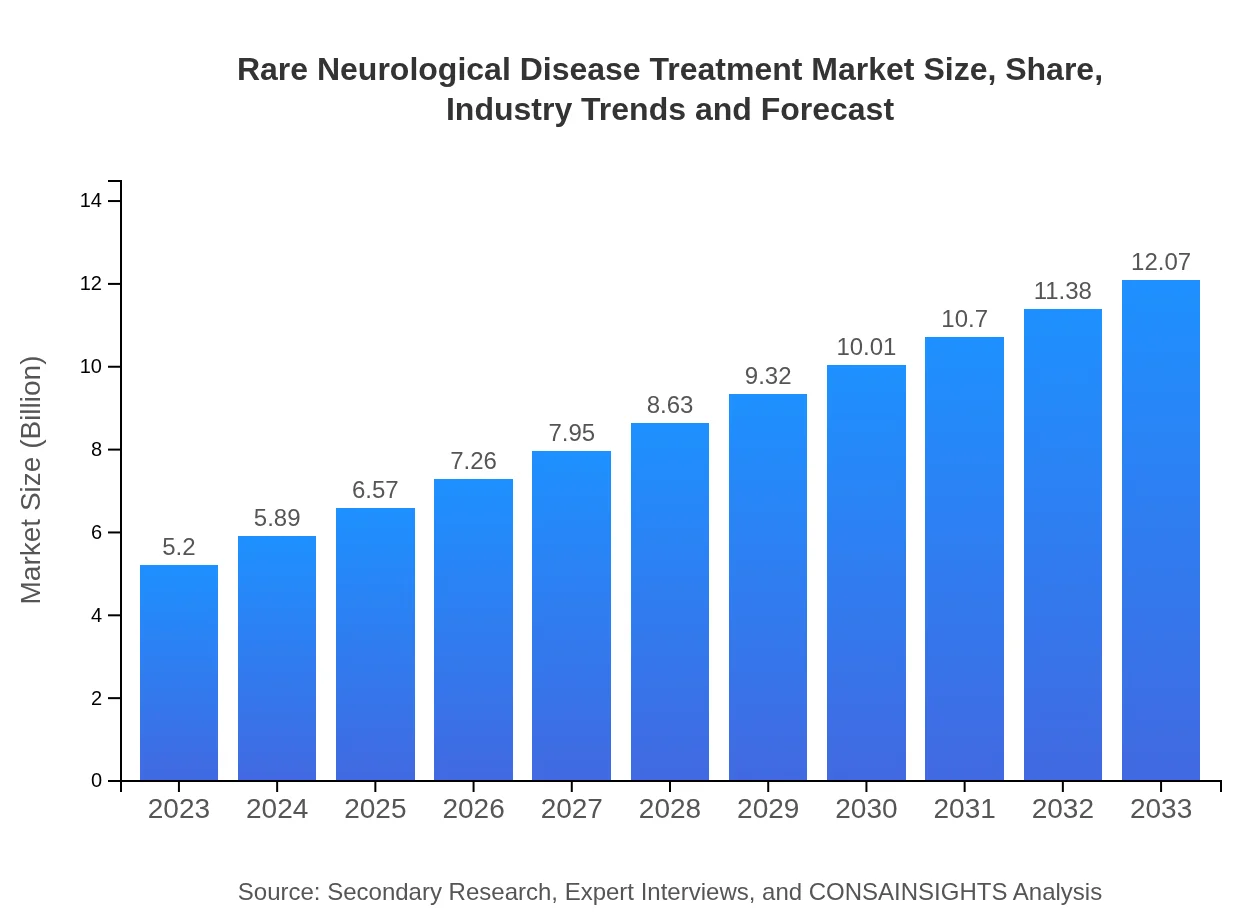
| 2023 Market Size | $5.20 Billion |
| CAGR (2023-2033) | 8.5% |
| 2033 Market Size | $12.07 Billion |
| Top Companies | Biogen, Novartis, Orphazyme, Sarepta Therapeutics |
| Last Modified Date | 31 January 2026 |
Rare Neurological Disease Treatment Market Overview
Customize Rare Neurological Disease Treatment Market Report market research report
- ✔ Get in-depth analysis of Rare Neurological Disease Treatment market size, growth, and forecasts.
- ✔ Understand Rare Neurological Disease Treatment's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Rare Neurological Disease Treatment
What is the Market Size & CAGR of Rare Neurological Disease Treatment market in 2033?
Rare Neurological Disease Treatment Industry Analysis
Rare Neurological Disease Treatment Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Rare Neurological Disease Treatment Market Analysis Report by Region
Europe Rare Neurological Disease Treatment Market Report:
The European market is expected to rise significantly from $1.61 billion in 2023 to $3.73 billion by 2033. Healthcare initiatives addressing rare diseases, coupled with supportive regulatory frameworks and funding for innovative research, drive this growth.Asia Pacific Rare Neurological Disease Treatment Market Report:
The Asia Pacific region's Rare Neurological Disease Treatment market is expected to grow from $0.98 billion in 2023 to $2.27 billion by 2033. Increased healthcare expenditure, rising awareness about rare diseases, and improving access to treatment facilities in countries like Japan and China contribute to this growth.North America Rare Neurological Disease Treatment Market Report:
North America is projected to witness substantial growth, increasing from $1.84 billion in 2023 to $4.28 billion by 2033. The region's advanced healthcare system, strong focus on research and development, and high adoption rates of new therapies are crucial factors fueling market expansion.South America Rare Neurological Disease Treatment Market Report:
In South America, the market is anticipated to expand from $0.31 billion in 2023 to $0.71 billion by 2033. Growing healthcare infrastructure and rising prevalence of neurological disorders are significant growth drivers in this region, especially in larger markets such as Brazil and Argentina.Middle East & Africa Rare Neurological Disease Treatment Market Report:
The Middle East and Africa market is projected to grow from $0.46 billion in 2023 to $1.08 billion by 2033. Increasing awareness of rare neurological diseases and improving healthcare infrastructure in countries like Saudi Arabia and South Africa are key contributors to market growth.Tell us your focus area and get a customized research report.
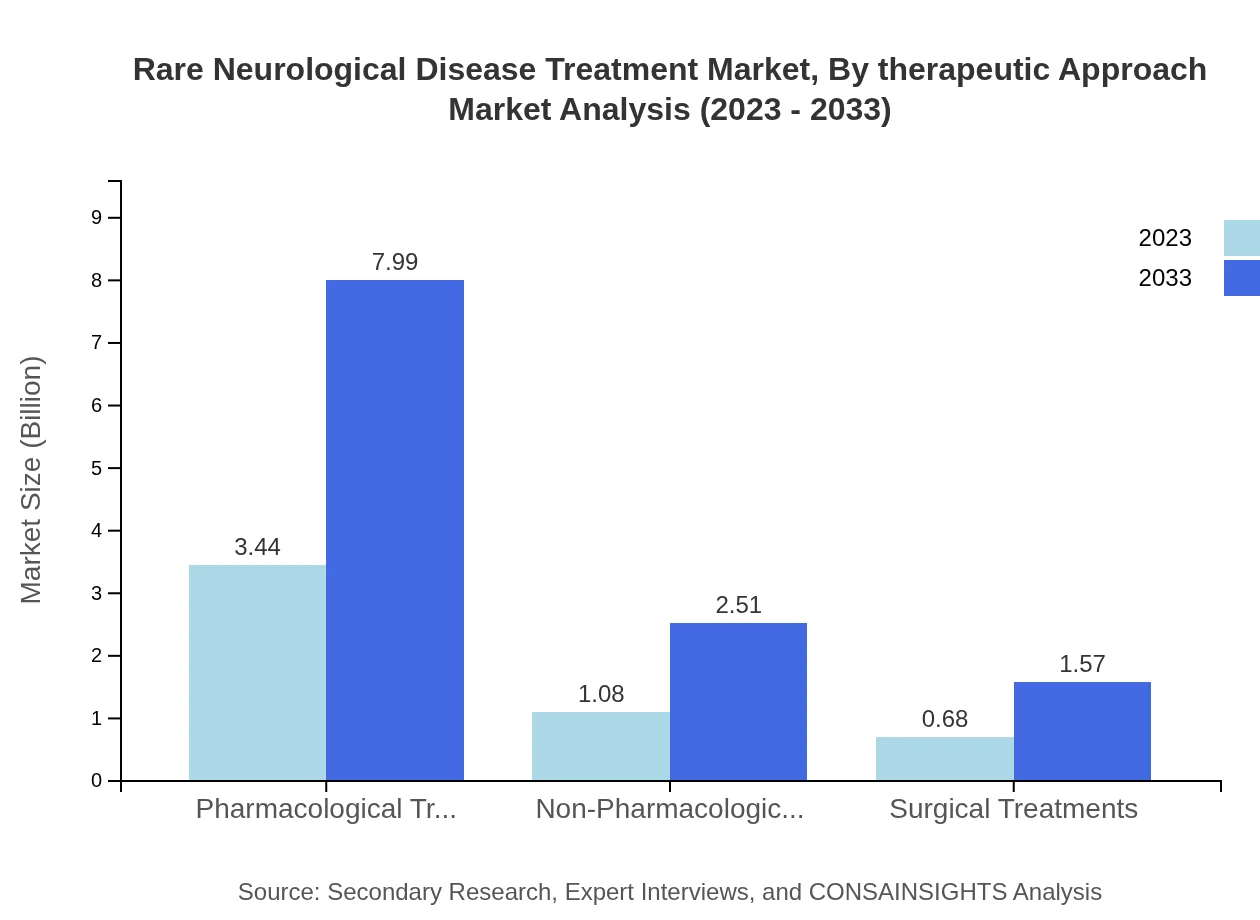
Rare Neurological Disease Treatment Market Analysis By Therapeutic Approach
In 2023, pharmacological treatments account for a significant share, valued at $3.44 billion and expected to double to $7.99 billion by 2033. Non-pharmacological treatment approaches are also on the rise, increasing from $1.08 billion in 2023 to $2.51 billion in 2033. Innovative therapies, especially those that leverage technology, are enhancing treatment efficacy and patient outcomes.
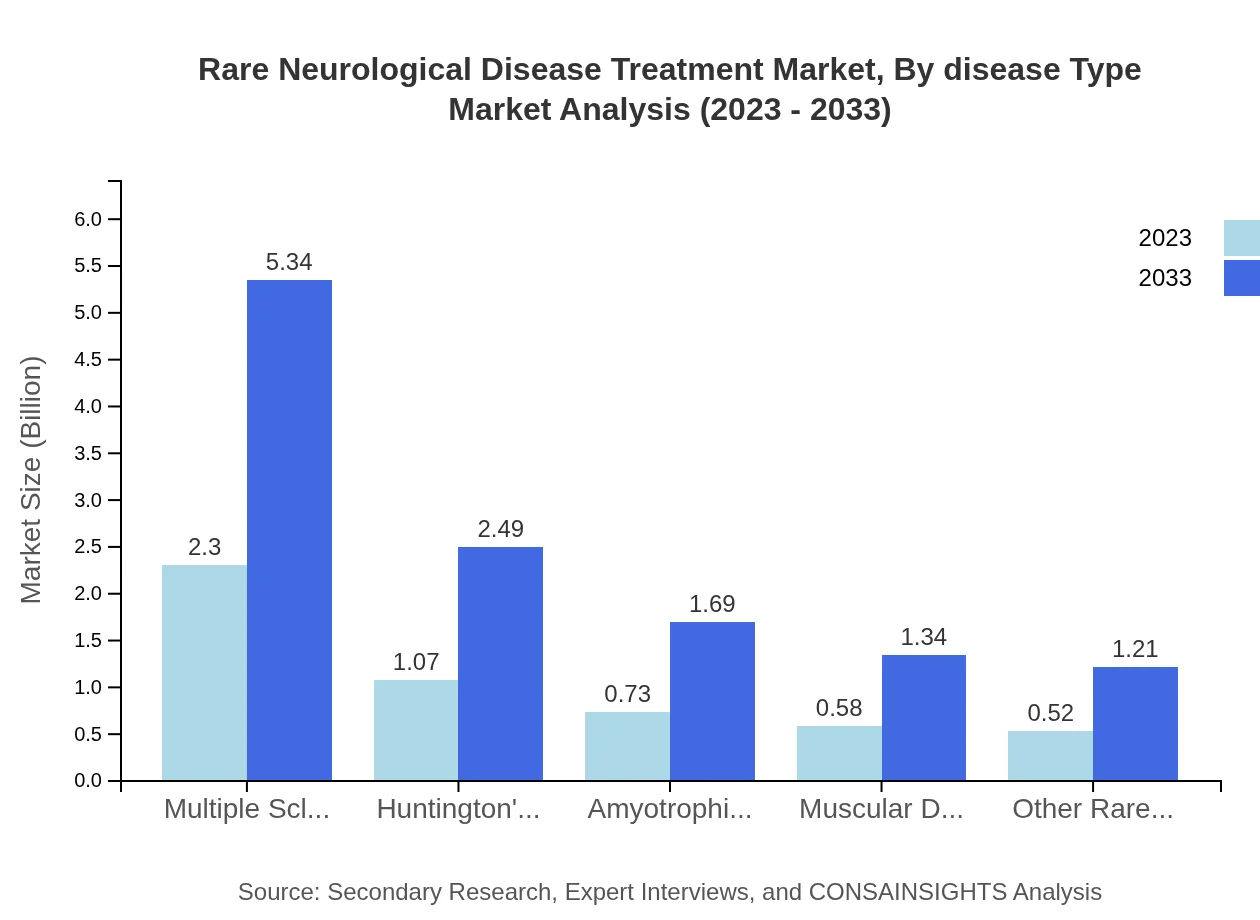
Rare Neurological Disease Treatment Market Analysis By Disease Type
Multiple Sclerosis remains the leading segment with a market size of $2.30 billion in 2023, expected to reach $5.34 billion by 2033, capturing nearly 44% of the total market share. Other diseases, such as Huntington's Disease and Amyotrophic Lateral Sclerosis, contribute steadily, with their treatment markets projected to experience similar double-digit growth rates.
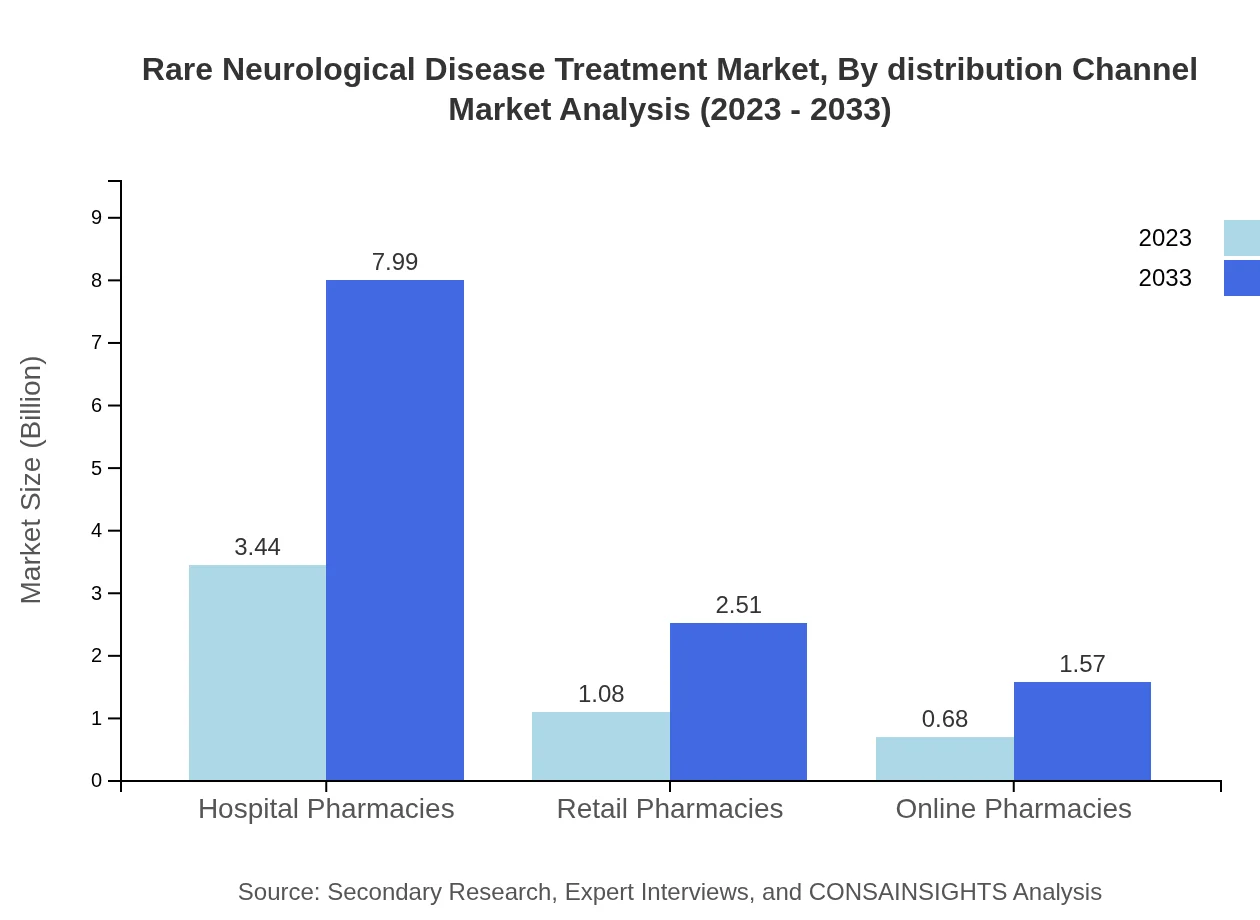
Rare Neurological Disease Treatment Market Analysis By Distribution Channel
Hospital pharmacies dominate the distribution channel sector, projected to grow from $3.44 billion in 2023 to $7.99 billion by 2033, maintaining a market share of 66.22%. Retail pharmacies and online platforms are also pivotal, growing significantly as consumers and healthcare providers seek more accessible treatment options.
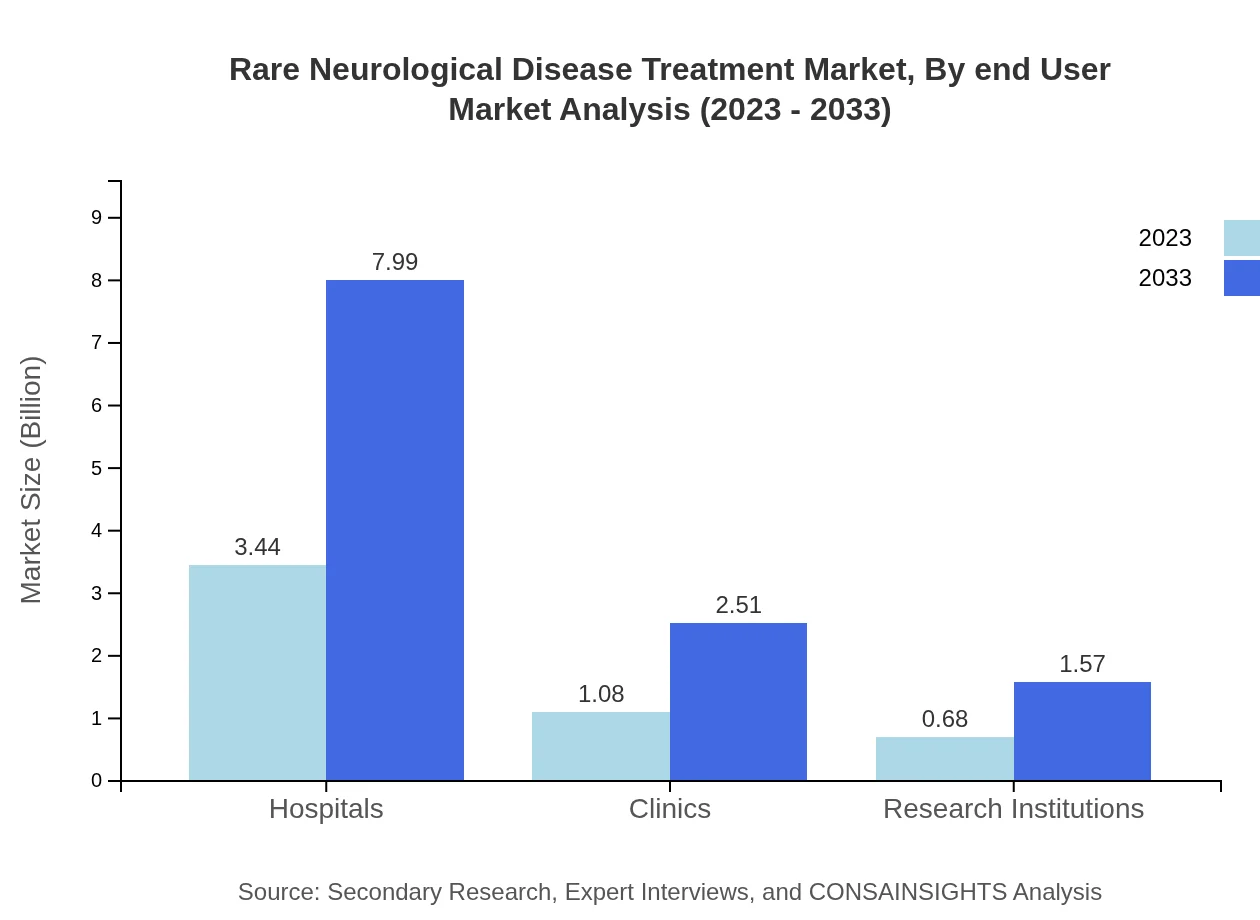
Rare Neurological Disease Treatment Market Analysis By End User
The market for end-users shows hospitals at the forefront, accounting for approximately 66.22% of the market share in 2023. Clinics will grow steadily, from $1.08 billion in 2023 to $2.51 billion by 2033, highlighting the importance of multi-faceted treatment approaches that integrate various healthcare environments.
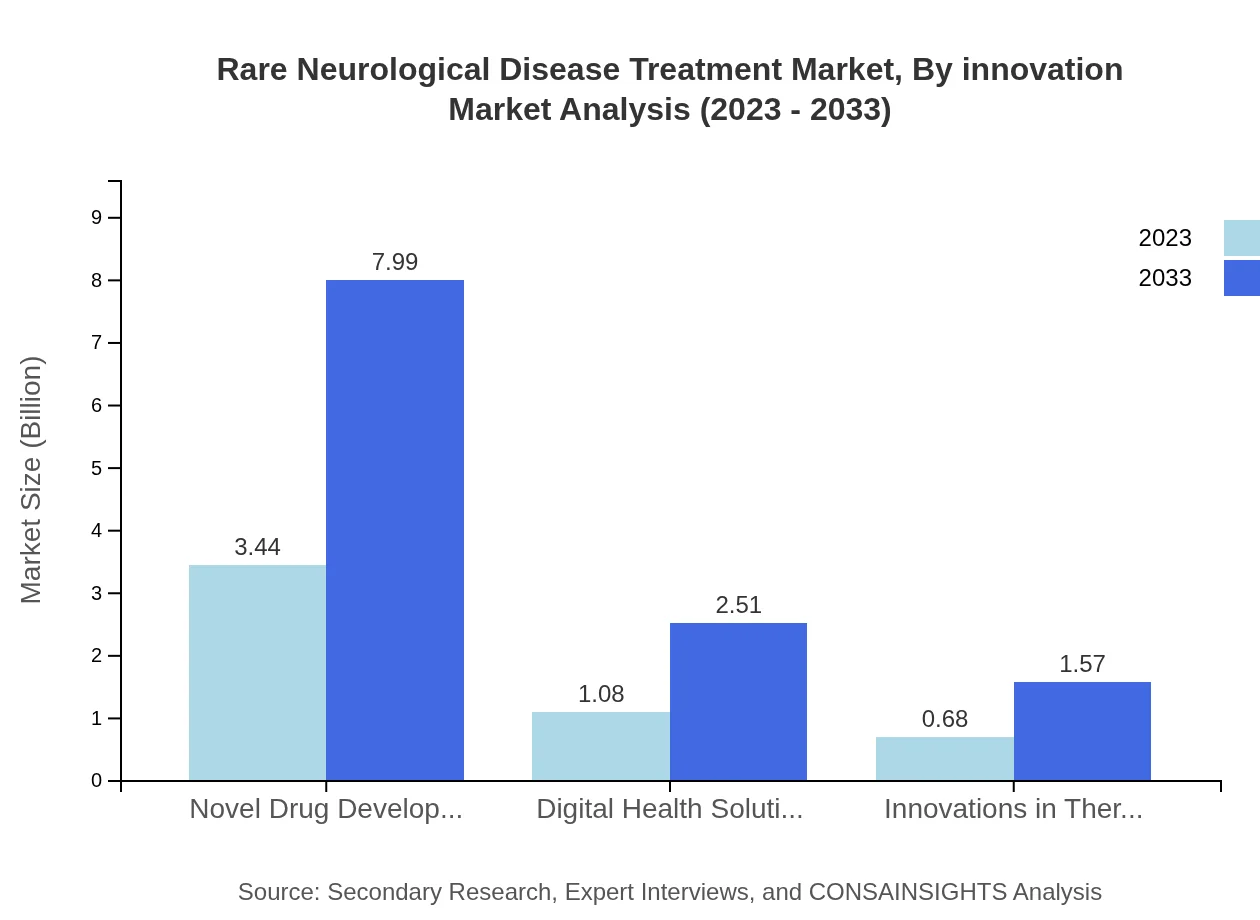
Rare Neurological Disease Treatment Market Analysis By Innovation
Innovation, particularly in digital health solutions and therapeutic device advancements, is shaping the future of the Rare Neurological Disease Treatment market. This segment is poised to rise from $0.68 billion in 2023 to $1.57 billion by 2033, emphasizing the significant role of technology in enhancing treatment delivery and patient engagement.
Rare Neurological Disease Treatment Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Rare Neurological Disease Treatment Industry
Biogen:
Biogen is a leading biopharmaceutical company specializing in therapies for neurological conditions, particularly Multiple Sclerosis treatment, and pioneering innovative research in neurodegenerative diseases.Novartis:
Novartis develops advanced therapies for several rare neurological disorders and invests significantly in research, resulting in groundbreaking treatments that address unmet medical needs.Orphazyme:
A biotechnology company focused on developing treatments for rare diseases, Orphazyme is known for its work on heat-shock protein modulation in neurodegenerative conditions.Sarepta Therapeutics:
Sarepta is recognized for developing revolutionary gene therapy solutions targeting muscular dystrophies and other rare neurological diseases.We're grateful to work with incredible clients.









FAQs
What is the market size of rare neurological disease treatment?
The rare neurological disease treatment market is valued at approximately $5.2 billion in 2023, with a projected CAGR of 8.5%. This growth underscores the increasing demand for innovative therapies addressing these complex conditions.
What are the key market players or companies in this industry?
Key players in the rare neurological disease treatment market include major pharmaceutical firms and biotechnology companies innovating in drug development and therapeutic solutions, focusing on unmet medical needs.
What are the primary factors driving the growth in the industry?
Growth is primarily driven by rising prevalence of rare neurological disorders, advancements in drug development technologies, increased funding for research, and a growing focus on personalized medicine, enhancing treatment outcomes.
Which region is the fastest Growing in the rare neurological disease treatment market?
The Asia Pacific region is the fastest-growing market, anticipated to expand from $0.98 billion in 2023 to $2.27 billion by 2033, driven by increased healthcare investments and rising awareness of neurological disorders.
Does ConsaInsights provide customized market report data for the industry?
Yes, ConsaInsights offers customized market research reports tailored to specific client needs, providing detailed insights that enhance strategic planning and decision-making in the rare neurological disease treatment sector.
What deliverables can I expect from this market research project?
Deliverables from the market research project include comprehensive reports, data analytics, market forecasts, competitive analysis, and insights into emerging trends and consumer behavior within the rare neurological disease treatment market.
What are the market trends of rare neurological disease treatment?
Current market trends include a rise in collaborative research initiatives, growing adoption of digital health technologies, increased market entry of novel therapies, and heightened focus on patient-centric drug development approaches.