Real World Evidence Solutions Market Report
Published Date: 31 January 2026 | Report Code: real-world-evidence-solutions
Real World Evidence Solutions Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Real World Evidence Solutions market, including current trends, market sizes, and future forecasts from 2023 to 2033, for insightful decision-making and strategic planning.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
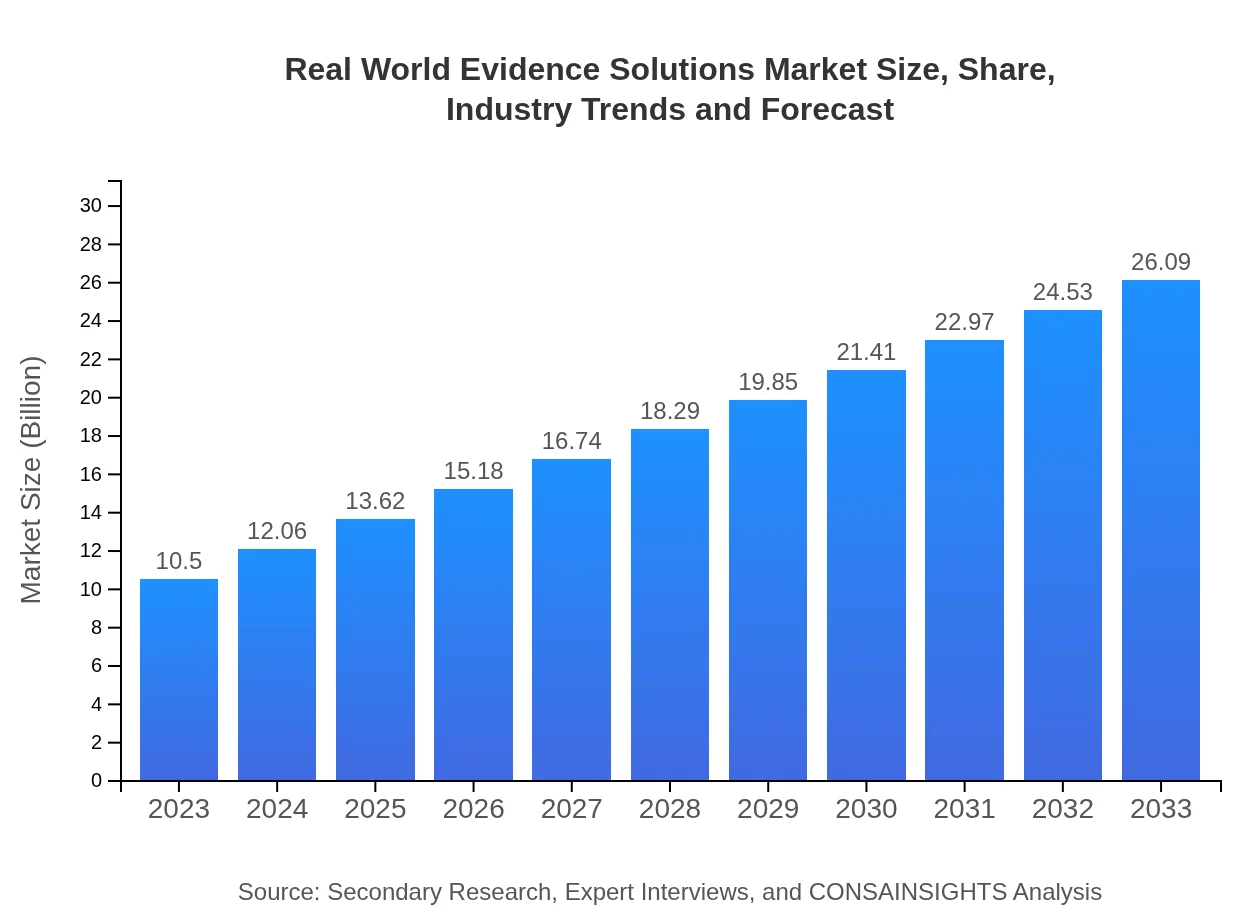
| 2023 Market Size | $10.50 Billion |
| CAGR (2023-2033) | 9.2% |
| 2033 Market Size | $26.09 Billion |
| Top Companies | IBM Watson Health, Flatiron Health, Optum, Aetion |
| Last Modified Date | 31 January 2026 |
Real World Evidence Solutions Market Overview
Customize Real World Evidence Solutions Market Report market research report
- ✔ Get in-depth analysis of Real World Evidence Solutions market size, growth, and forecasts.
- ✔ Understand Real World Evidence Solutions's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Real World Evidence Solutions
What is the Market Size & CAGR of Real World Evidence Solutions market in 2023?
Real World Evidence Solutions Industry Analysis
Real World Evidence Solutions Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Real World Evidence Solutions Market Analysis Report by Region
Europe Real World Evidence Solutions Market Report:
Europe’s RWE market is forecasted to increase from $3.47 billion in 2023 to $8.62 billion by 2033, supported by stringent regulatory requirements and a growing emphasis on value-based healthcare solutions, paving the way for more proactive decision-making based on real-world data.Asia Pacific Real World Evidence Solutions Market Report:
In Asia Pacific, the RWE market is forecasted to grow from $1.90 billion in 2023 to $4.71 billion by 2033. The region's growth is driven by the rising healthcare expenditure, adoption of technology in health systems, and increasing regulatory support for using real-world data in pharmacovigilance and market access.North America Real World Evidence Solutions Market Report:
North America leads the Real World Evidence Solutions market, growing from $3.60 billion in 2023 to $8.94 billion by 2033. Factors such as the increased use of big data, advancements in healthcare technologies, and supportive regulatory frameworks are propelling this growth.South America Real World Evidence Solutions Market Report:
The South American market is expected to expand from $0.23 billion in 2023 to $0.57 billion by 2033. Growth in this region is largely due to an increased focus on health data analytics and the implementation of healthcare reforms aimed at improving patient outcomes.Middle East & Africa Real World Evidence Solutions Market Report:
The Middle East and Africa region is projected to grow from $1.30 billion in 2023 to $3.24 billion by 2033. Growth opportunities stem from rising investments in healthcare infrastructure, digital health initiatives, and a growing recognition of the importance of real-world evidence.Tell us your focus area and get a customized research report.
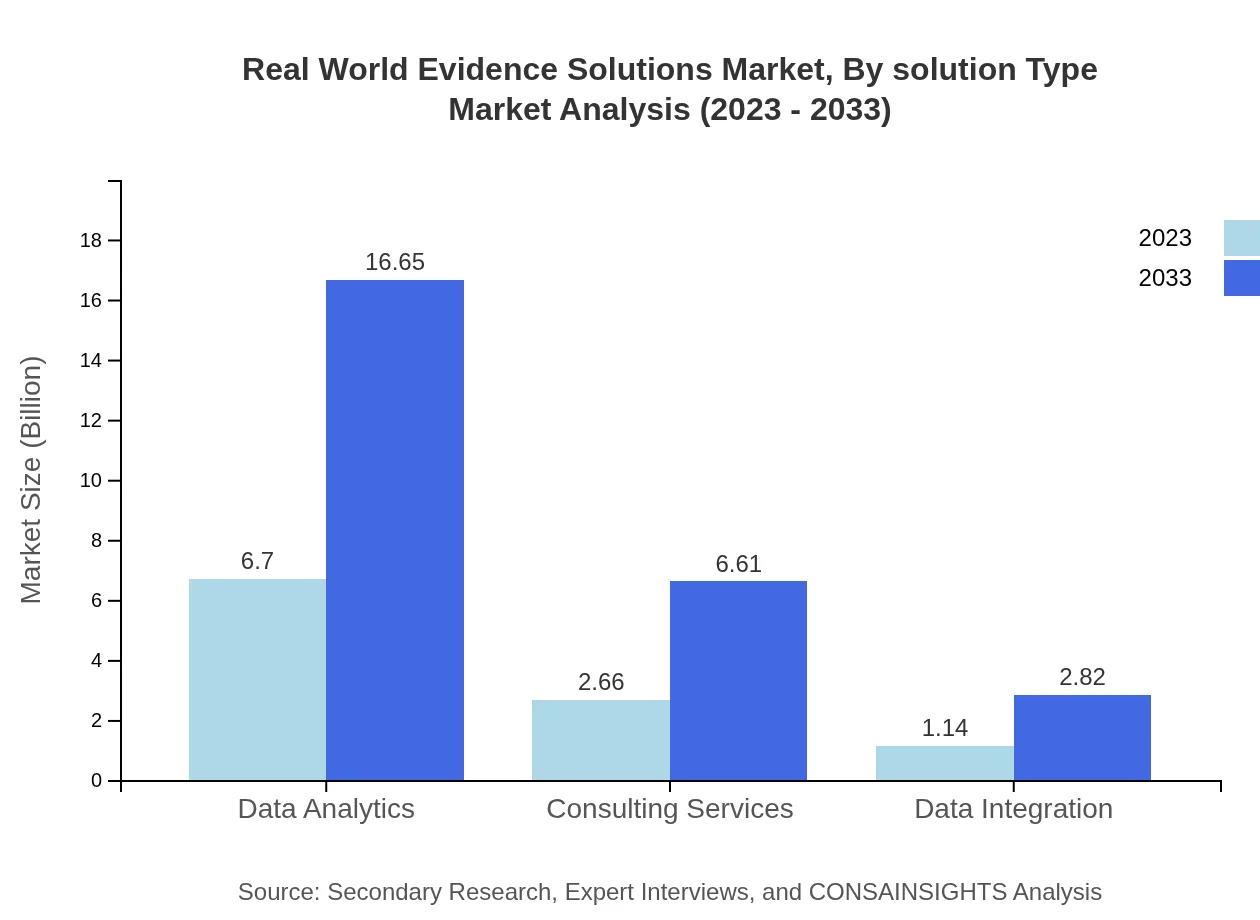
Real World Evidence Solutions Market Analysis By Solution Type
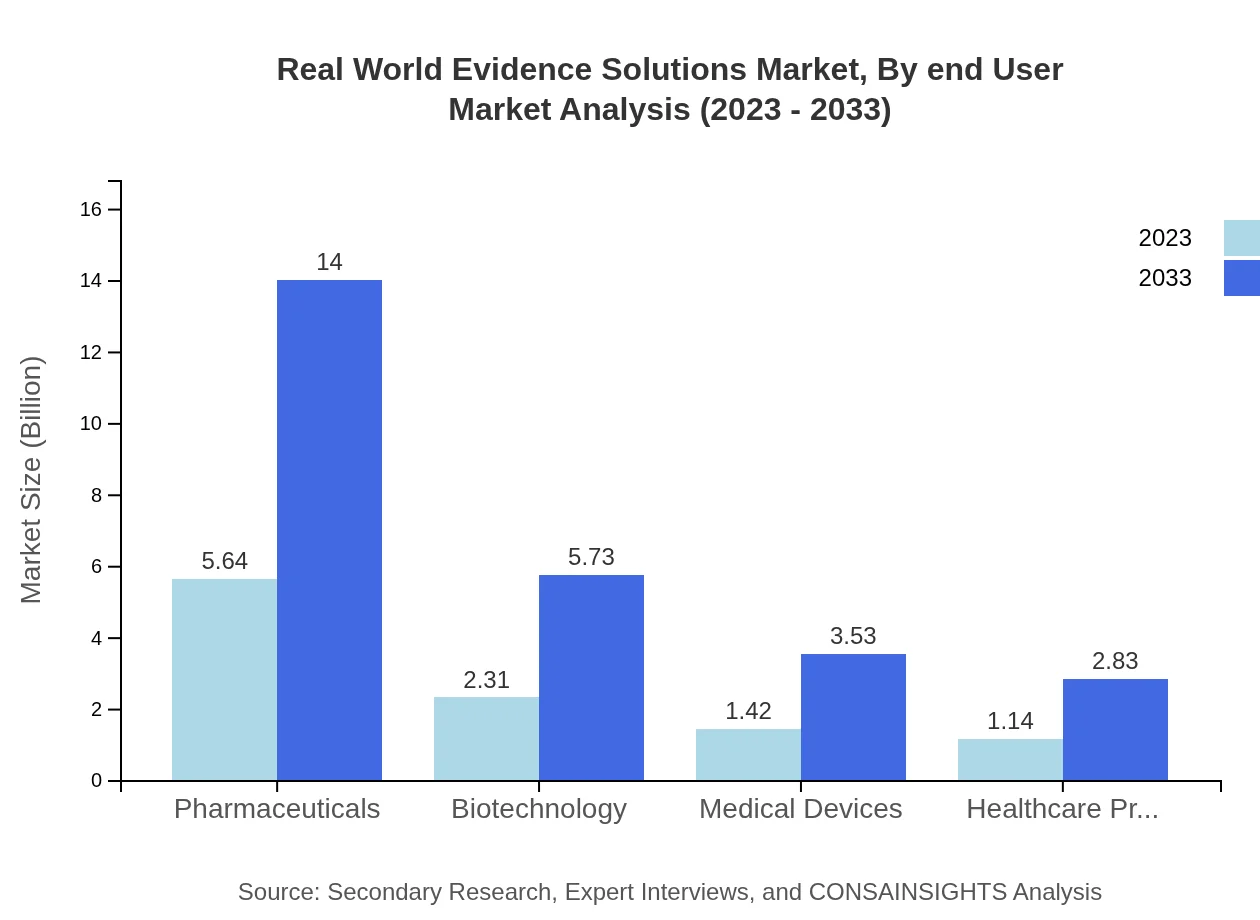
The Real-World Evidence Solutions market, segmented by solution type, shows significant growth potential. The Pharmaceuticals segment is a major player, projected to increase from $5.64 billion in 2023 to $14.00 billion by 2033, holding a steady market share. The Biotechnology segment is also notable, expanding from $2.31 billion to $5.73 billion over the same period.
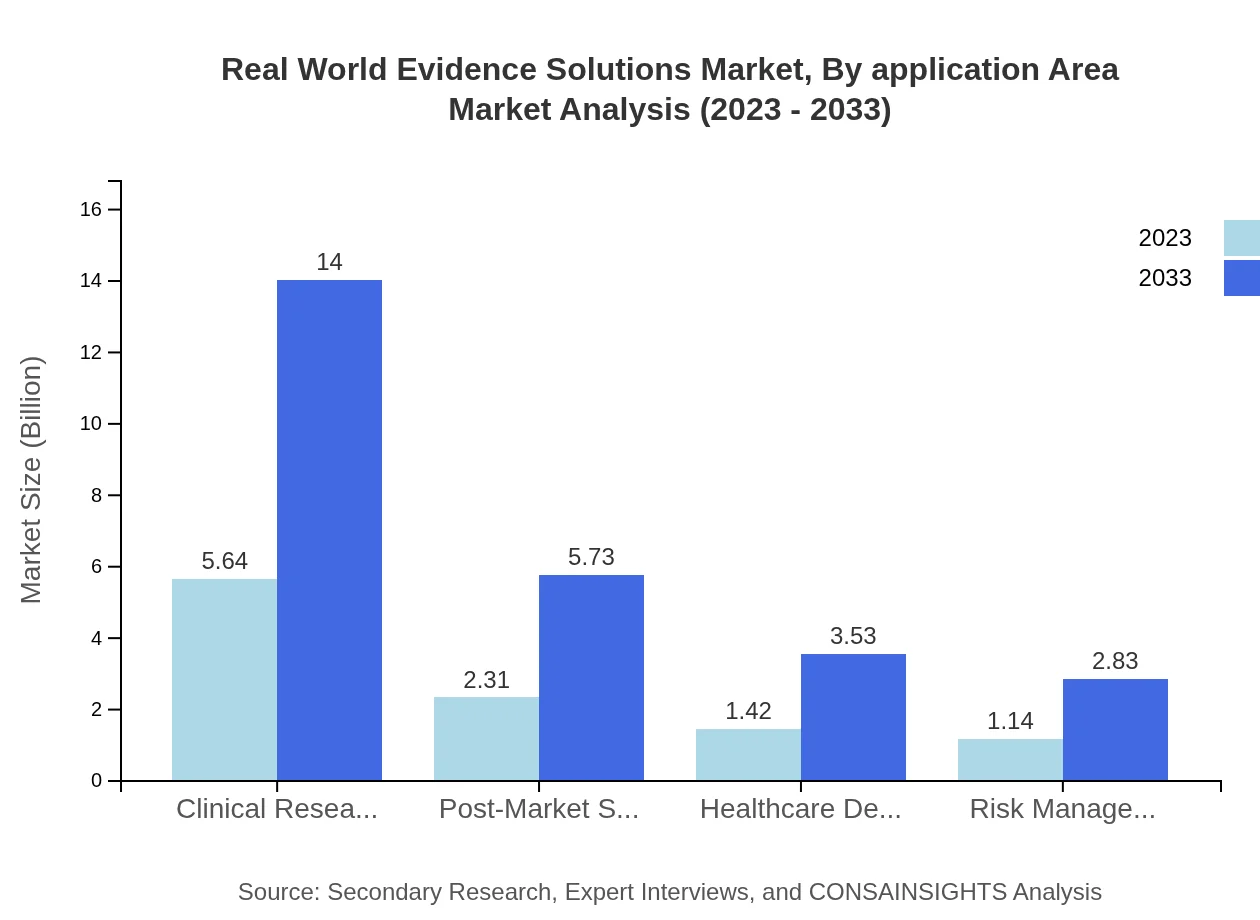
Real World Evidence Solutions Market Analysis By Application Area
In terms of application areas, clinical research leads the market with an expected growth from $5.64 billion in 2023 to $14.00 billion by 2033, leveraging real-world evidence for drug efficacy studies. Other areas such as Healthcare Decision Making and Risk Management are also showing robust potential, ensuring informed choices in treatment pathways.
Real World Evidence Solutions Market Analysis By End User
The end-user segmentation reveals that Healthcare Providers occupy a significant share, projected to grow from $1.14 billion to $2.83 billion by 2033. The pharmaceutical companies' increasing reliance on RWE for market access and compliance creates a fertile environment for growth.
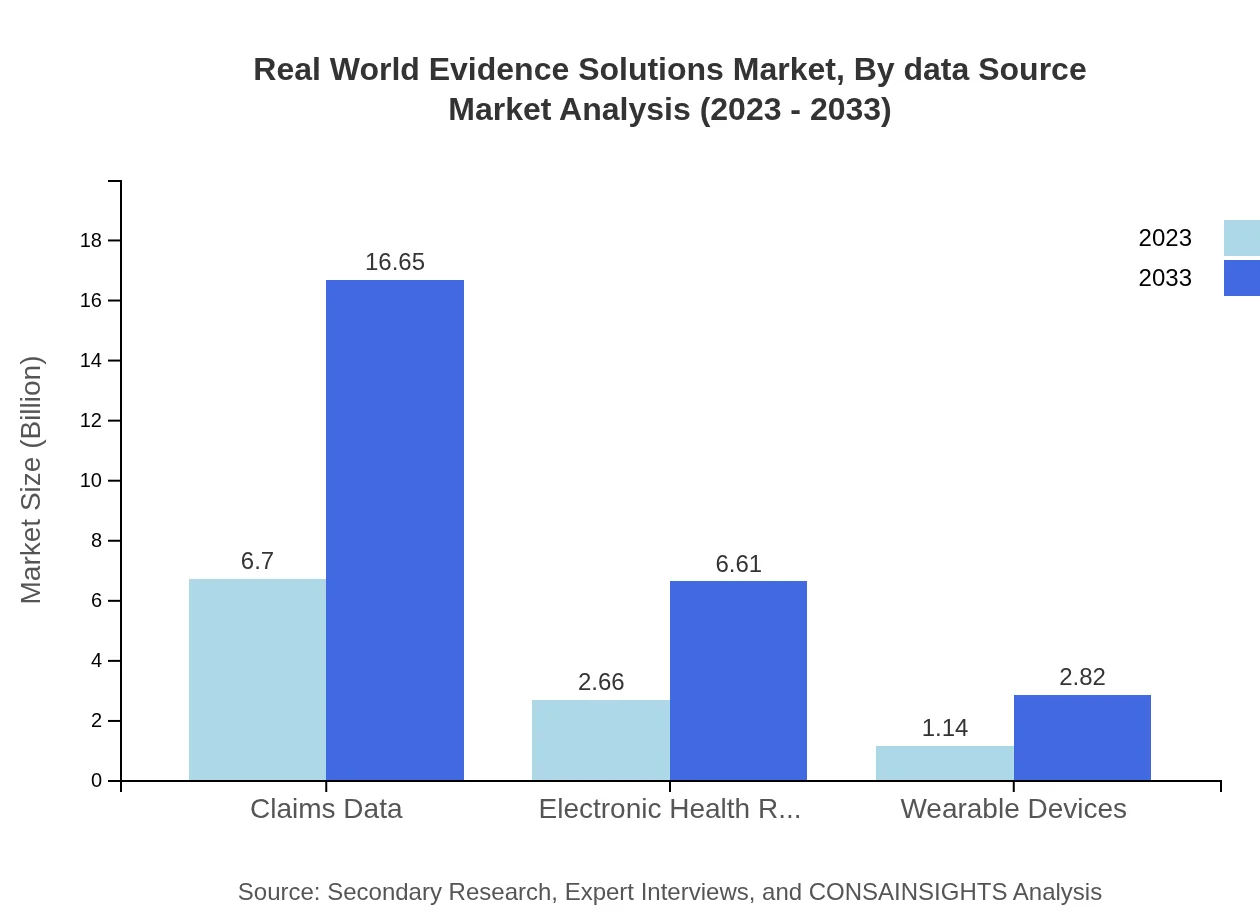
Real World Evidence Solutions Market Analysis By Data Source
Data Sources in the Real World Evidence Solutions market, particularly Claims Data, represent the largest segment, with market growth expected from $6.70 billion in 2023 to $16.65 billion by 2033. This highlights the rising importance of comprehensive data analytics and integration in developing effective RWE methodologies.
Real World Evidence Solutions Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Real World Evidence Solutions Industry
IBM Watson Health:
IBM Watson Health leverages advanced analytics and cognitive computing to facilitate real-world evidence generation in healthcare, enhancing outcomes and cost efficiencies.Flatiron Health:
Flatiron Health specializes in oncology data, providing an integrated technology platform for real-world data solutions that drive actionable insights for cancer care.Optum:
A leading health services company, Optum uses data and analytics to support healthcare decisions and improve care delivery, making substantial contributions to RWE methodologies.Aetion:
Aetion provides a platform that enables stakeholders to analyze real-world data and deliver evidence on the value of treatments, supporting regulatory submissions and market access.We're grateful to work with incredible clients.









FAQs
What is the market size of Real-World Evidence Solutions?
The market size of Real-World Evidence Solutions is projected to reach approximately $10.5 billion by 2033, with a CAGR of 9.2% from 2023. This growth is indicative of the increasing demand for data-driven healthcare solutions.
What are the key market players or companies in this Real-World Evidence Solutions industry?
Key players in the Real-World Evidence Solutions market include major pharmaceutical companies, technology providers specializing in healthcare analytics, and contract research organizations. Their innovations and collaborations shape the landscape of this rapidly evolving industry.
What are the primary factors driving the growth in the Real-World Evidence Solutions industry?
Primary factors driving growth include the increasing adoption of data analytics in healthcare, the need for cost-effective solutions, regulatory support for real-world data utilization, and the demand for personalized medicine, fostering a deeper understanding of patient outcomes.
Which region is the fastest Growing in the Real-World Evidence Solutions?
The Asia Pacific region is the fastest-growing market for Real-World Evidence Solutions, projected to grow from $1.90 billion in 2023 to $4.71 billion by 2033. This growth is attributed to advancements in healthcare infrastructure and increasing investments.
Does ConsaInsights provide customized market report data for the Real-World Evidence Solutions industry?
Yes, ConsaInsights offers customized market report data tailored to specific client needs within the Real-World Evidence Solutions industry. This includes detailed analysis, region-specific insights, and segment data, ensuring comprehensive research support.
What deliverables can I expect from this Real-World Evidence Solutions market research project?
Deliverables include detailed market reports, trend analysis, competitive landscape assessments, customized segments analysis, and actionable insights on growth opportunities in the Real-World Evidence Solutions market tailored to client objectives.
What are the market trends of Real-World Evidence Solutions?
Current market trends include a shift towards advanced data analytics, growth in electronic health records, increased use of real-world data in regulatory submissions, and a focus on cost-effective healthcare solutions, shaping the industry's future landscape.