Recombinant Chinese Hamster Ovary Cell Cho Hepatitis B Vaccine Market Report
Published Date: 31 January 2026 | Report Code: recombinant-chinese-hamster-ovary-cell-cho-hepatitis-b-vaccine
Recombinant Chinese Hamster Ovary Cell Cho Hepatitis B Vaccine Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Recombinant Chinese Hamster Ovary Cell Cho Hepatitis B Vaccine market, offering insights into market size, growth projections, segmentation, and regional analysis for the forecast period from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
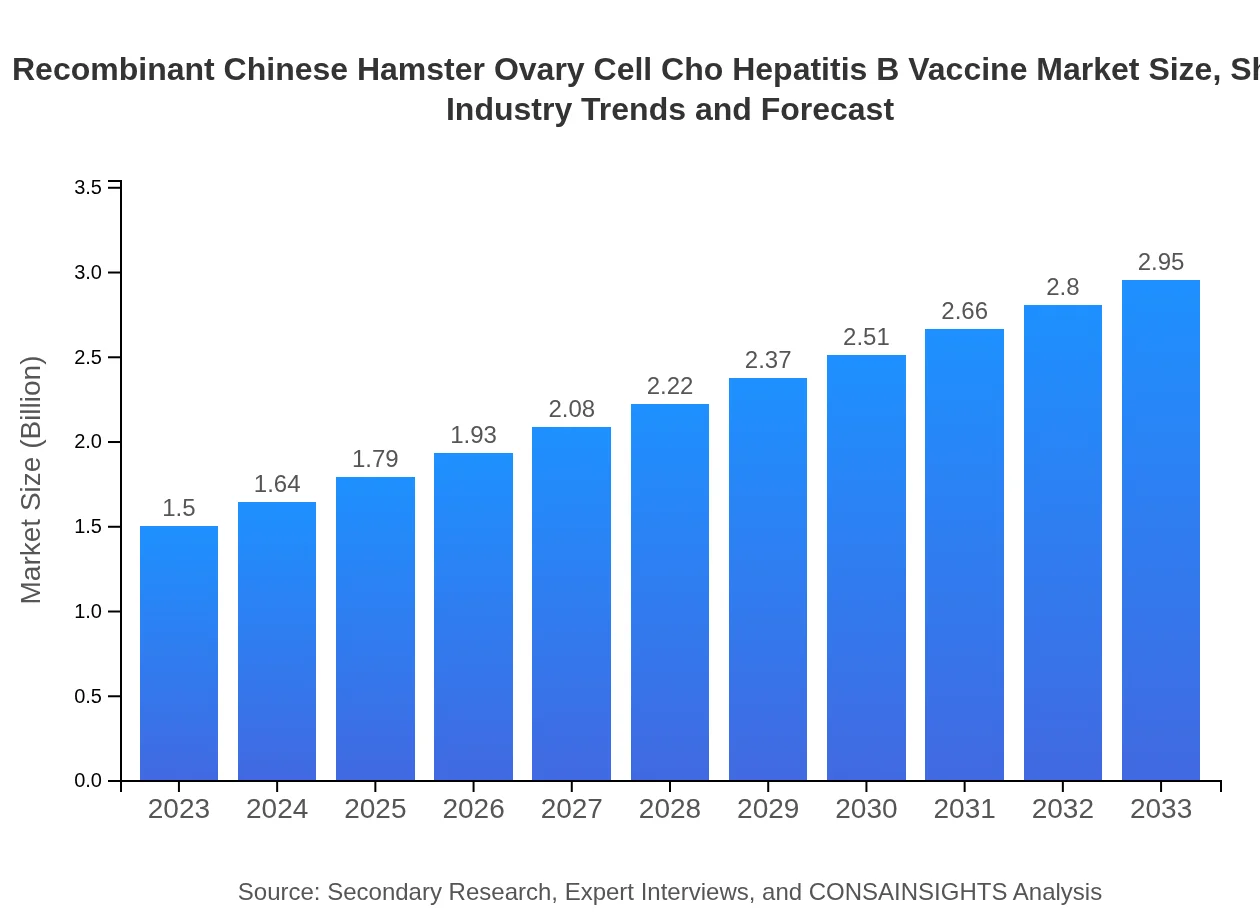
| 2023 Market Size | $1.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $2.95 Billion |
| Top Companies | GSK (GlaxoSmithKline), Novo Nordisk, Merck & Co., Pfizer Inc. |
| Last Modified Date | 31 January 2026 |
Recombinant Chinese Hamster Ovary Cell Cho Hepatitis B Vaccine Market Overview
Customize Recombinant Chinese Hamster Ovary Cell Cho Hepatitis B Vaccine Market Report market research report
- ✔ Get in-depth analysis of Recombinant Chinese Hamster Ovary Cell Cho Hepatitis B Vaccine market size, growth, and forecasts.
- ✔ Understand Recombinant Chinese Hamster Ovary Cell Cho Hepatitis B Vaccine's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Recombinant Chinese Hamster Ovary Cell Cho Hepatitis B Vaccine
What is the Market Size & CAGR of Recombinant Chinese Hamster Ovary Cell Cho Hepatitis B Vaccine market in 2023?
Recombinant Chinese Hamster Ovary Cell Cho Hepatitis B Vaccine Industry Analysis
Recombinant Chinese Hamster Ovary Cell Cho Hepatitis B Vaccine Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Recombinant Chinese Hamster Ovary Cell Cho Hepatitis B Vaccine Market Analysis Report by Region
Europe Recombinant Chinese Hamster Ovary Cell Cho Hepatitis B Vaccine Market Report:
Europe's market, currently valued at $0.40 billion, is expected to grow to $0.78 billion by 2033, driven by strict healthcare regulations and well-established healthcare systems promoting preventive health measures.Asia Pacific Recombinant Chinese Hamster Ovary Cell Cho Hepatitis B Vaccine Market Report:
In Asia Pacific, the market is expected to grow from $0.29 billion in 2023 to $0.56 billion in 2033, driven by urbanization, improved healthcare systems, and increasing awareness of hepatitis vaccination.North America Recombinant Chinese Hamster Ovary Cell Cho Hepatitis B Vaccine Market Report:
North America exhibits robust growth, with the market set to rise from $0.52 billion in 2023 to $1.01 billion in 2033, fueled by advanced healthcare infrastructure and high vaccination rates.South America Recombinant Chinese Hamster Ovary Cell Cho Hepatitis B Vaccine Market Report:
The South American market, valued at $0.13 billion in 2023, is projected to reach $0.26 billion by 2033, supported by governmental health initiatives and rising health insurance coverage.Middle East & Africa Recombinant Chinese Hamster Ovary Cell Cho Hepatitis B Vaccine Market Report:
In the Middle East and Africa, the vaccine market is set to grow from $0.17 billion in 2023 to $0.33 billion in 2033, influenced by rising healthcare investments and efforts to combat infectious diseases.Tell us your focus area and get a customized research report.
Recombinant Chinese Hamster Ovary Cell Cho Hepatitis B Vaccine Market Analysis By Product
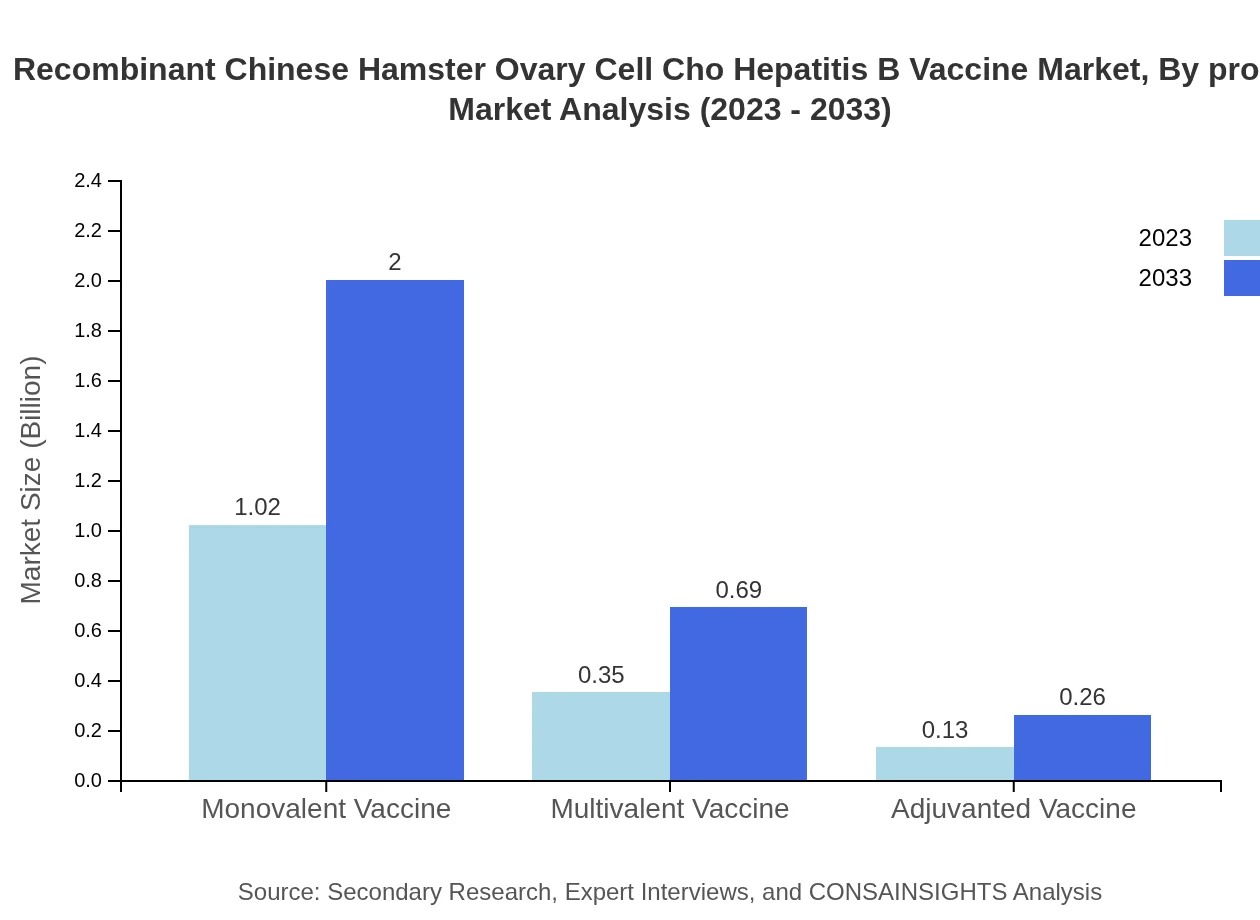
The CHO Hepatitis B Vaccine market is primarily segmented by product into monovalent, multivalent, and adjuvanted vaccines. As of 2023, the monovalent vaccines dominate the market, generating a size of $1.02 billion, which is expected to increase to $2 billion by 2033, maintaining a consistent market share of about 67.72%. Multivalent vaccines, with a market size of $0.35 billion in 2023, will grow to $0.69 billion by 2033, while adjuvanted vaccines remain dedicated to niche applications, showing gradual growth.
Recombinant Chinese Hamster Ovary Cell Cho Hepatitis B Vaccine Market Analysis By Application
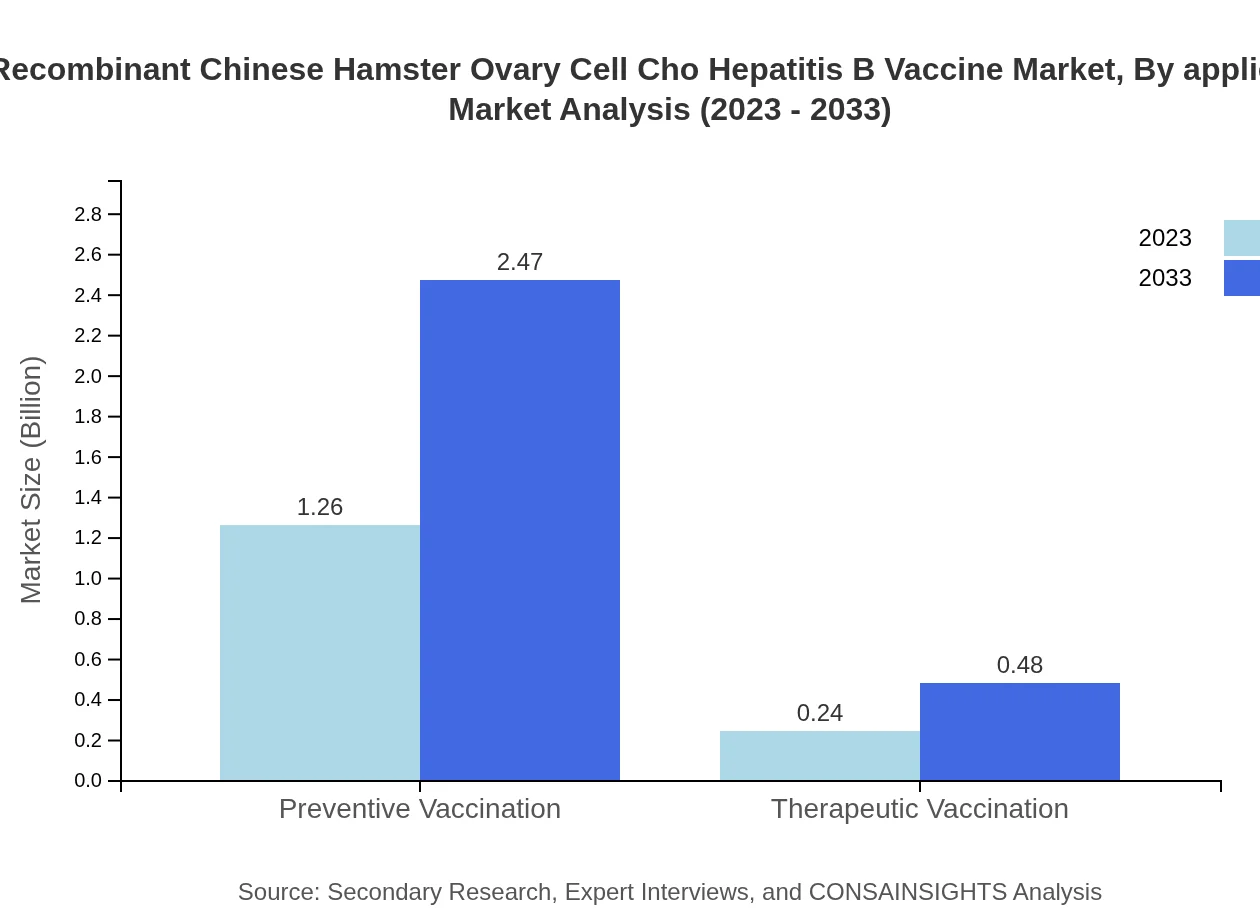
Applications of the CHO Hepatitis B Vaccine are categorized into preventive and therapeutic vaccinations. Preventive vaccination dominates the application segment, with a size of $1.26 billion in 2023, expanding to $2.47 billion by 2033. This segment holds an 83.84% market share, while therapeutic vaccinations represent a smaller market with steady growth.
Recombinant Chinese Hamster Ovary Cell Cho Hepatitis B Vaccine Market Analysis By Distribution Channel
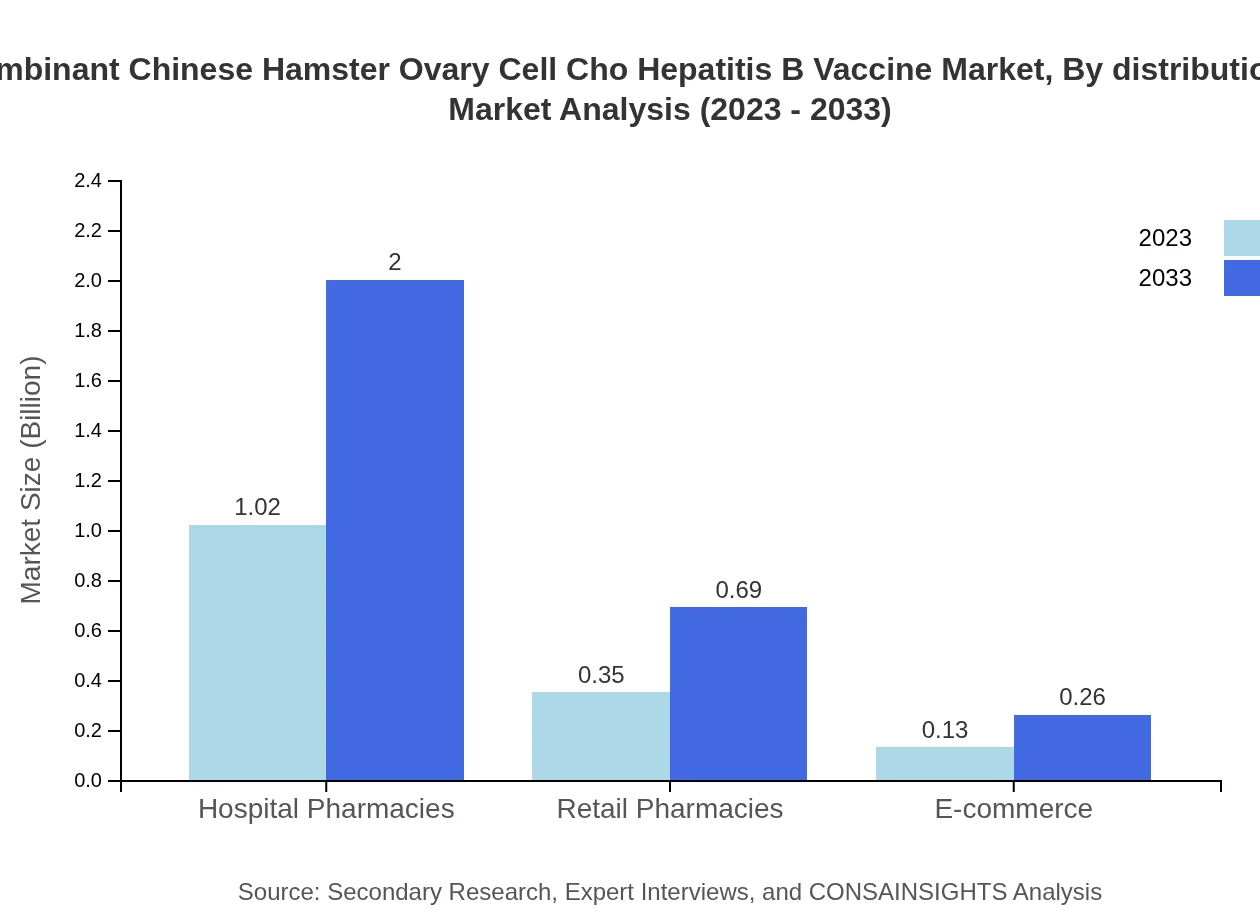
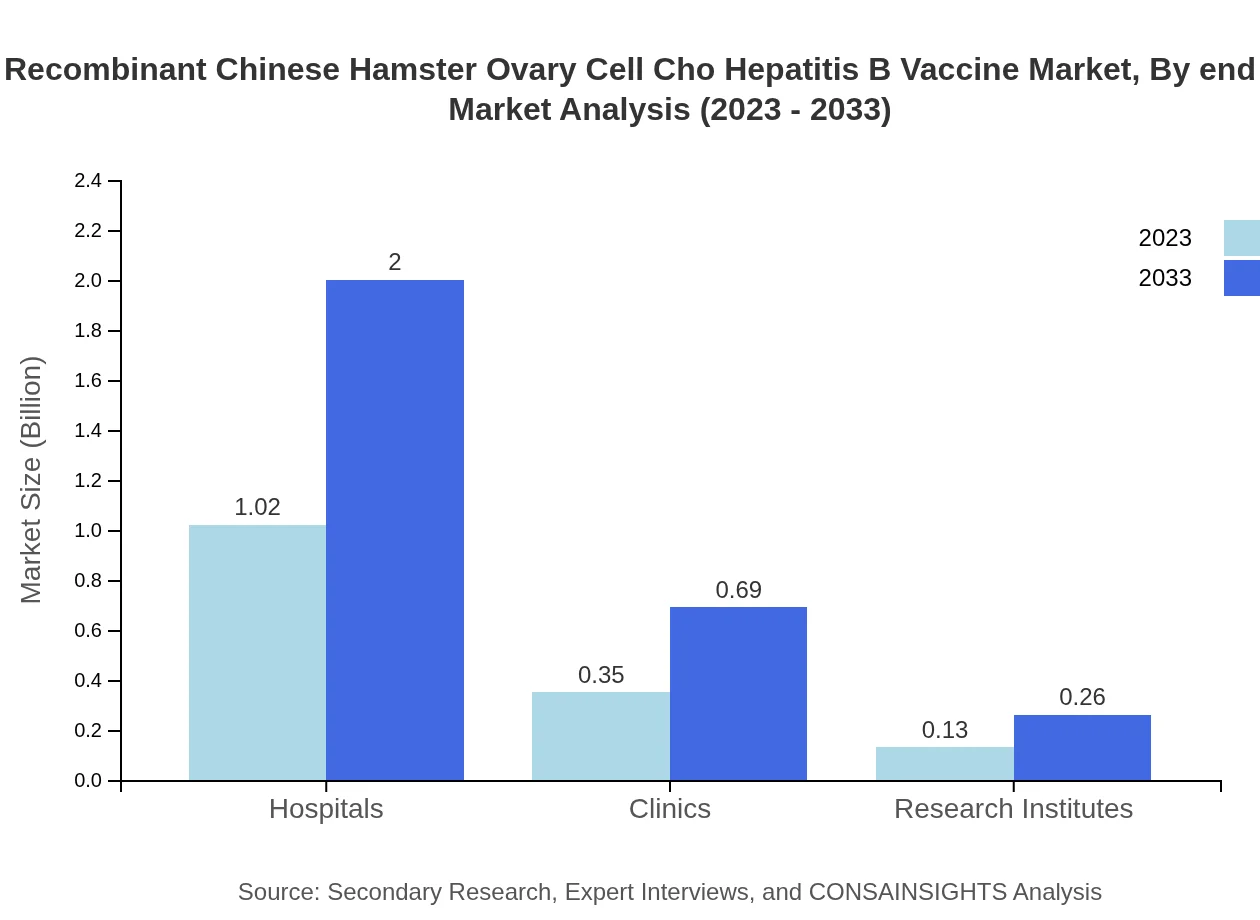
The distribution channels for the CHO Hepatitis B Vaccine include hospitals, clinics, pharmacies, and e-commerce platforms. Hospitals remain the leading distribution channel with a size of $1.02 billion in 2023, expected to increase to $2 billion by 2033. Clinics, representing a market size of $0.35 billion, will also see growth, while e-commerce is a growing segment capturing increasingly health-conscious consumers.
Recombinant Chinese Hamster Ovary Cell Cho Hepatitis B Vaccine Market Analysis By End User
End users of CHO Hepatitis B Vaccines fall within healthcare institutions and individual consumers. While hospitals and clinics capture a significant share, individual immunity needs drive market growth. As health awareness increases, more individuals seek vaccinations, particularly in emerging markets, thereby expanding total market size over the forecast period.
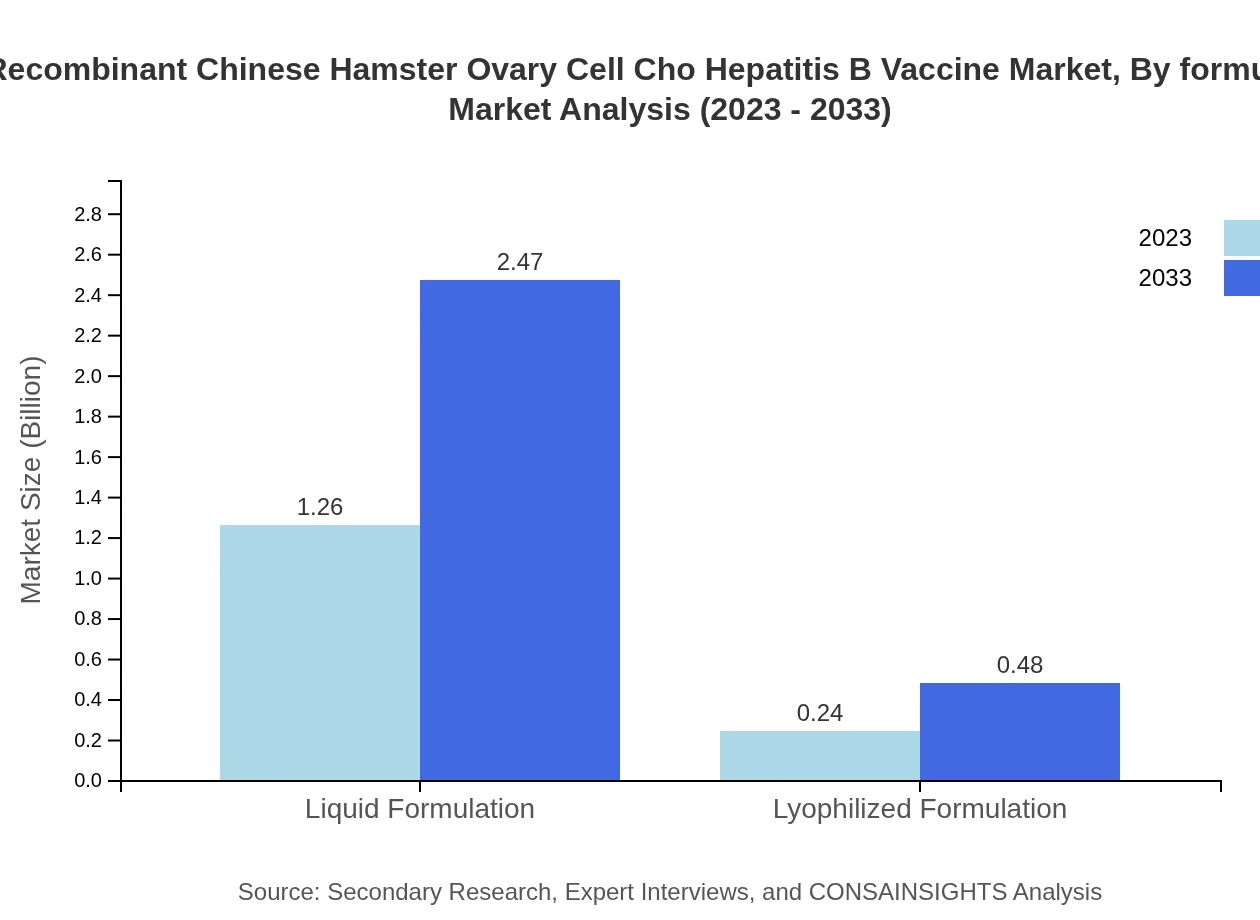
Recombinant Chinese Hamster Ovary Cell Cho Hepatitis B Vaccine Market Analysis By Formulation
Formulations of the CHO Hepatitis B Vaccine are divided into liquid and lyophilized forms. The liquid formulation enjoys a significant share, valued at $1.26 billion and expected to grow rapidly to $2.47 billion by 2033, supported by ease of administration and storage, while lyophilized formulations cater to specific needs of global markets.
Recombinant Chinese Hamster Ovary Cell Cho Hepatitis B Vaccine Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Recombinant Chinese Hamster Ovary Cell Cho Hepatitis B Vaccine Industry
GSK (GlaxoSmithKline):
GSK is a leading player in vaccine development and manufacturing, with a significant portfolio aimed at global vaccination against Hepatitis B.Novo Nordisk:
Novo Nordisk focuses on innovative therapeutic solutions and has expanded its reach into preventive vaccines, notably for Hepatitis B.Merck & Co.:
Merck is a major global healthcare company actively involved in the research and production of recombinant vaccines, including Hepatitis B.Pfizer Inc.:
Pfizer is at the forefront of biopharmaceutical advancements and has a robust vaccine segment, including the CHO Hepatitis B Vaccine line.We're grateful to work with incredible clients.









FAQs
What is the market size of recombinant Chinese hamster ovary cell (CHO) hepatitis B vaccine?
The global market size for recombinant Chinese hamster ovary cell (CHO) hepatitis B vaccine is projected to reach $1.5 billion by 2033, growing at a CAGR of 6.8% from its current valuation.
What are the key market players or companies in this recombinant CHO hepatitis B vaccine industry?
Key players in the recombinant CHO hepatitis B vaccine industry include major pharmaceutical companies that specialize in vaccines, biotech firms focusing on recombinant technologies, and companies investing in research and development for vaccine innovations.
What are the primary factors driving the growth in the recombinant CHO hepatitis B vaccine industry?
The growth in the recombinant CHO hepatitis B vaccine industry is driven by increasing incidences of hepatitis B, advancements in vaccine technology, and rising awareness about preventive healthcare measures across the global population.
Which region is the fastest Growing in the recombinant CHO hepatitis B vaccine market?
The Asia-Pacific region is the fastest-growing market for recombinant CHO hepatitis B vaccines, with market size estimated to grow from $0.29 billion in 2023 to $0.56 billion in 2033, representing significant growth potential.
Does ConsaInsights provide customized market report data for the recombinant CHO hepatitis B vaccine industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the recombinant CHO hepatitis B vaccine industry, providing insights focused on particular market segments or regions.
What deliverables can I expect from this recombinant CHO hepatitis B vaccine market research project?
Deliverables from the recombinant CHO hepatitis B vaccine market research project include comprehensive reports detailing market size, trends, competitive analysis, and forecasts tailored to stakeholder requirements.
What are the market trends of recombinant CHO hepatitis B vaccine?
Current market trends in the recombinant CHO hepatitis B vaccine sector include increasing adoption of preventive vaccination strategies, growth in liquid formulations, and a shift towards online sales channels for better accessibility.