Recombinant Coagulation Factors Market Report
Published Date: 31 January 2026 | Report Code: recombinant-coagulation-factors
Recombinant Coagulation Factors Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Recombinant Coagulation Factors market, covering key insights, trends, and forecasts for the period 2023 to 2033, including market size, segmentation, regional analysis, and the competitive landscape.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
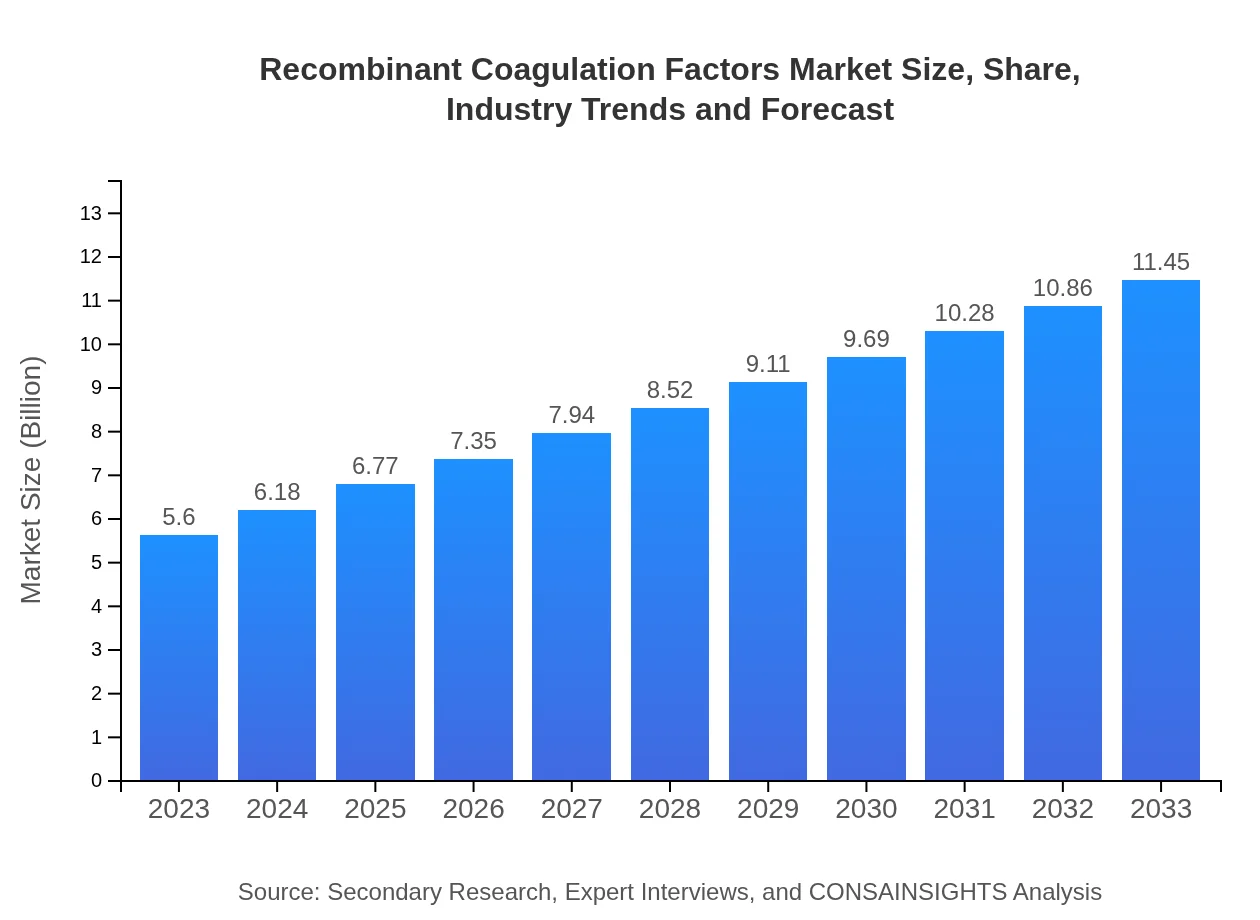
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 7.2% |
| 2033 Market Size | $11.45 Billion |
| Top Companies | Baxter International Inc., CSL Behring, Boehringer Ingelheim GmbH, Roche |
| Last Modified Date | 31 January 2026 |
Recombinant Coagulation Factors Market Overview
Customize Recombinant Coagulation Factors Market Report market research report
- ✔ Get in-depth analysis of Recombinant Coagulation Factors market size, growth, and forecasts.
- ✔ Understand Recombinant Coagulation Factors's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Recombinant Coagulation Factors
What is the Market Size & CAGR of Recombinant Coagulation Factors market in 2023?
Recombinant Coagulation Factors Industry Analysis
Recombinant Coagulation Factors Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Recombinant Coagulation Factors Market Analysis Report by Region
Europe Recombinant Coagulation Factors Market Report:
In Europe, the market size is projected to grow from $1.99 billion in 2023 to $4.07 billion by 2033. The region benefits from innovations in therapeutic approaches and supportive regulatory frameworks that foster the development of new treatments.Asia Pacific Recombinant Coagulation Factors Market Report:
In the Asia Pacific region, the Recombinant Coagulation Factors market is estimated to grow from $1.06 billion in 2023 to $2.16 billion by 2033, driven by improving healthcare infrastructure and rising acceptance of therapeutic advancements. Countries like China and India are increasingly adopting recombinant therapies.North America Recombinant Coagulation Factors Market Report:
North America holds a significant share of the market, estimated at $1.91 billion in 2023 and expected to reach $3.91 billion in 2033. This growth can be attributed to high healthcare spending, advanced research initiatives, and a well-established distribution network.South America Recombinant Coagulation Factors Market Report:
In South America, the market is projected to expand from $0.37 billion in 2023 to $0.75 billion by 2033. The growth is fueled by increasing investment in healthcare and rising awareness regarding effective hemophilia treatments across various countries in this region.Middle East & Africa Recombinant Coagulation Factors Market Report:
The Middle East and Africa region is anticipated to witness growth from $0.27 billion in 2023 to $0.55 billion by 2033. Factors contributing to this growth include enhanced healthcare policies, increasing awareness of bleeding disorders, and expansion of treatment facilities.Tell us your focus area and get a customized research report.
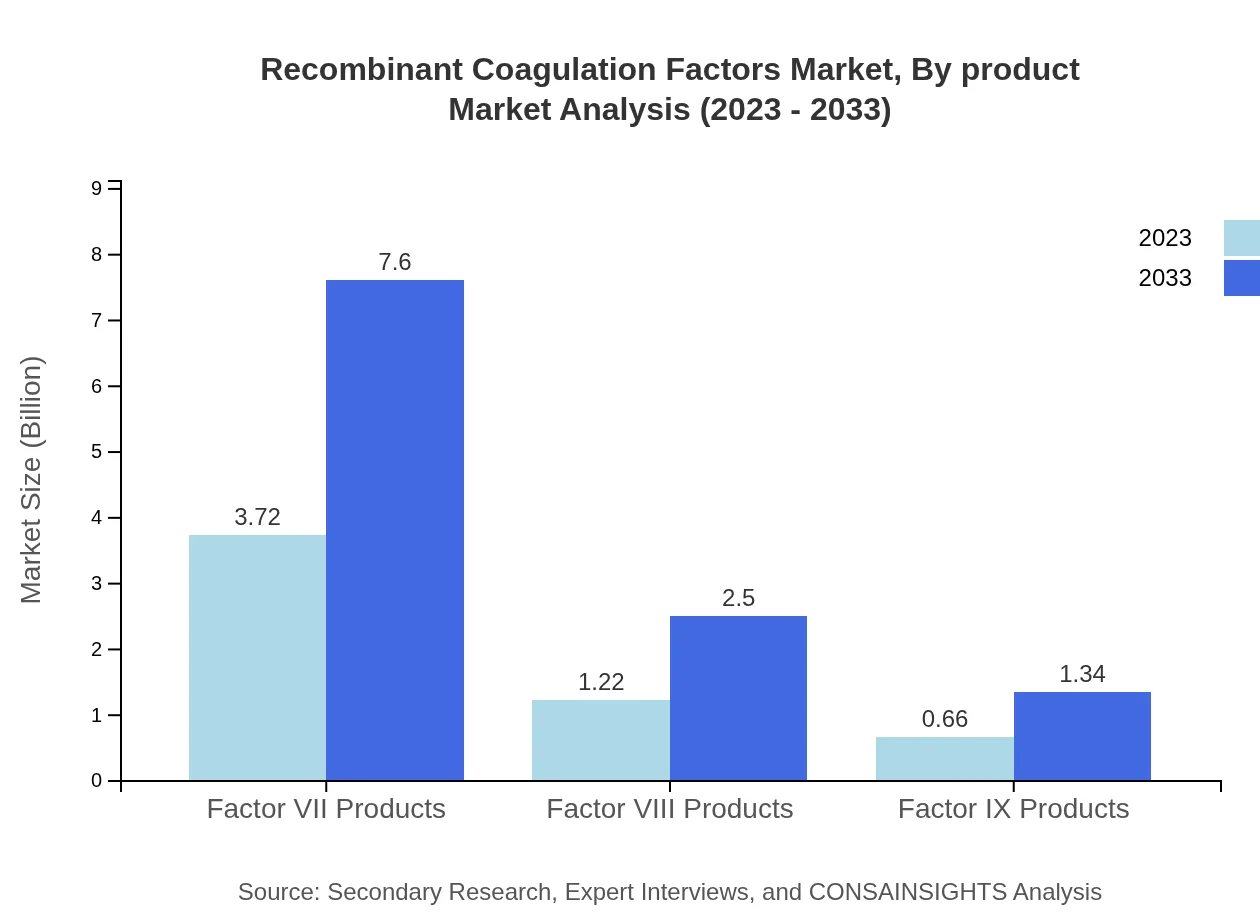
Recombinant Coagulation Factors Market Analysis By Product
In the product segment, Factor VII accounts for a significant market share, valued at $3.72 billion in 2023 and expected to reach $7.60 billion in 2033, representing a steady growth trend. Factor VIII is also significant, growing from $1.22 billion in 2023 to $2.50 billion by 2033, while Factor IX represents a smaller segment, increasing from $0.66 billion to $1.34 billion in the same timeframe.
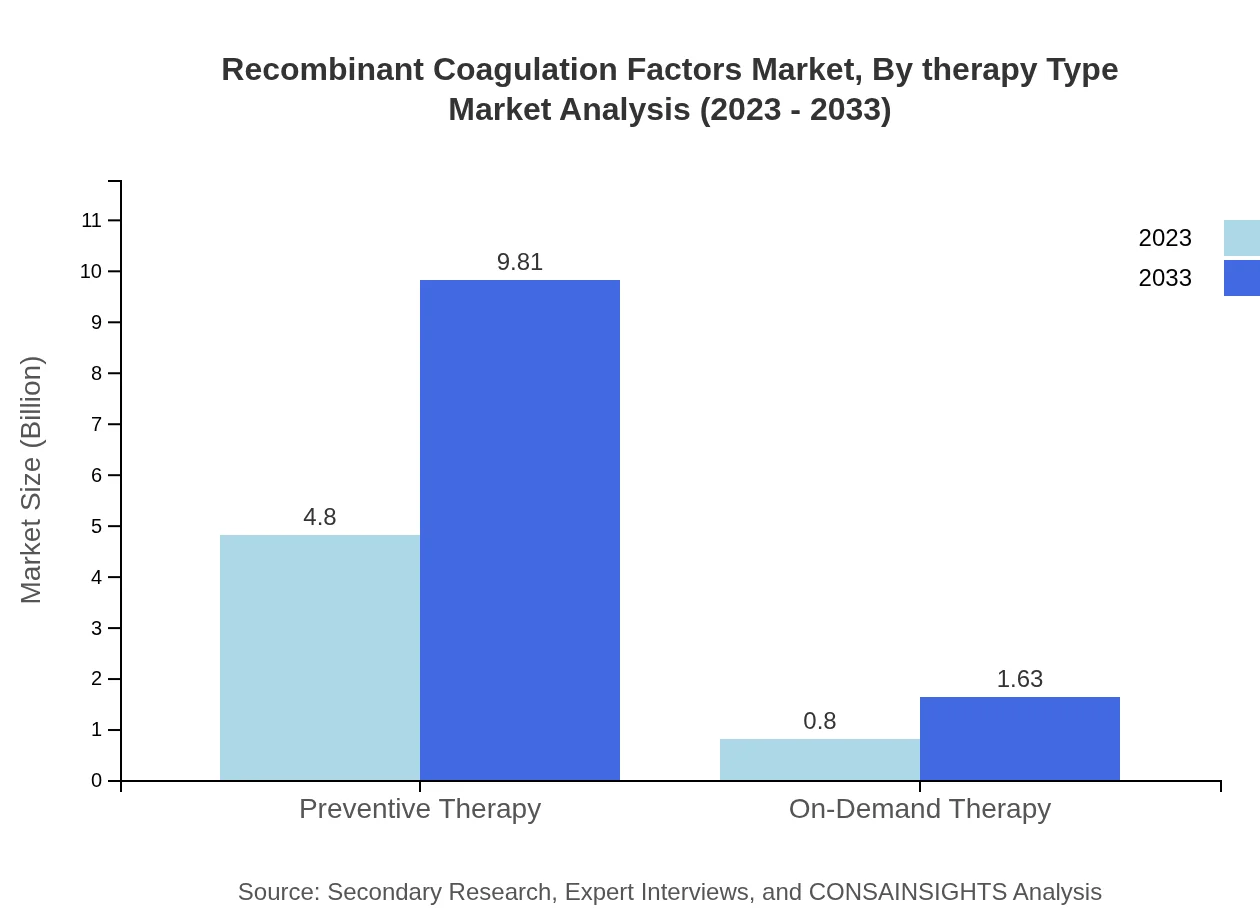
Recombinant Coagulation Factors Market Analysis By Therapy Type
Preventive therapy holds dominant market share at $4.80 billion in 2023, projected to rise to $9.81 billion by 2033. On-demand therapy, while growing at a slower pace from $0.80 billion to $1.63 billion, still plays a crucial role in treatment strategies for patients managing symptoms.
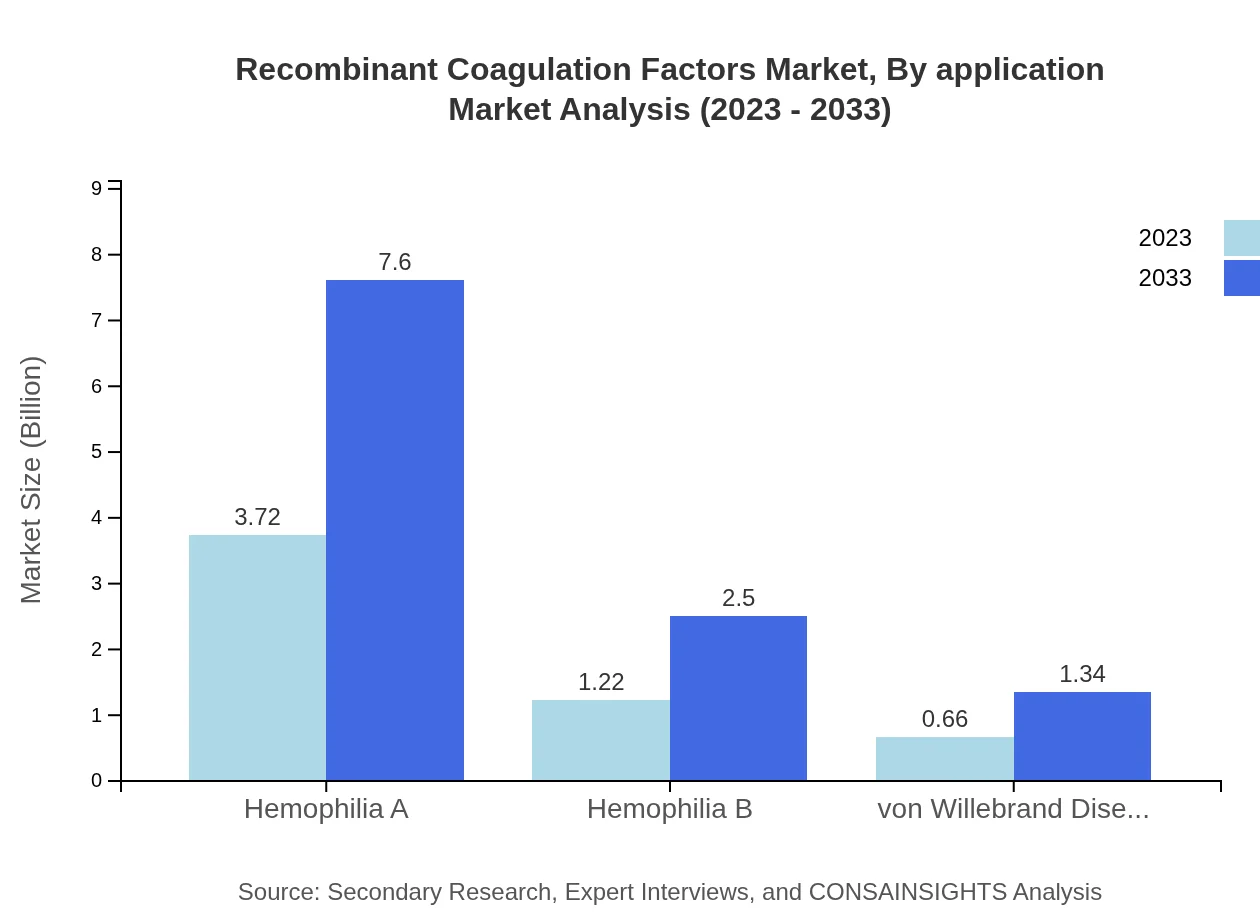
Recombinant Coagulation Factors Market Analysis By Application
Hemophilia A is the largest application segment, starting at $3.72 billion in 2023 and estimated to reach $7.60 billion by 2033. Hemophilia B and Von Willebrand disease are also relevant applications, expected to grow from $1.22 billion to $2.50 billion and from $0.66 billion to $1.34 billion respectively.
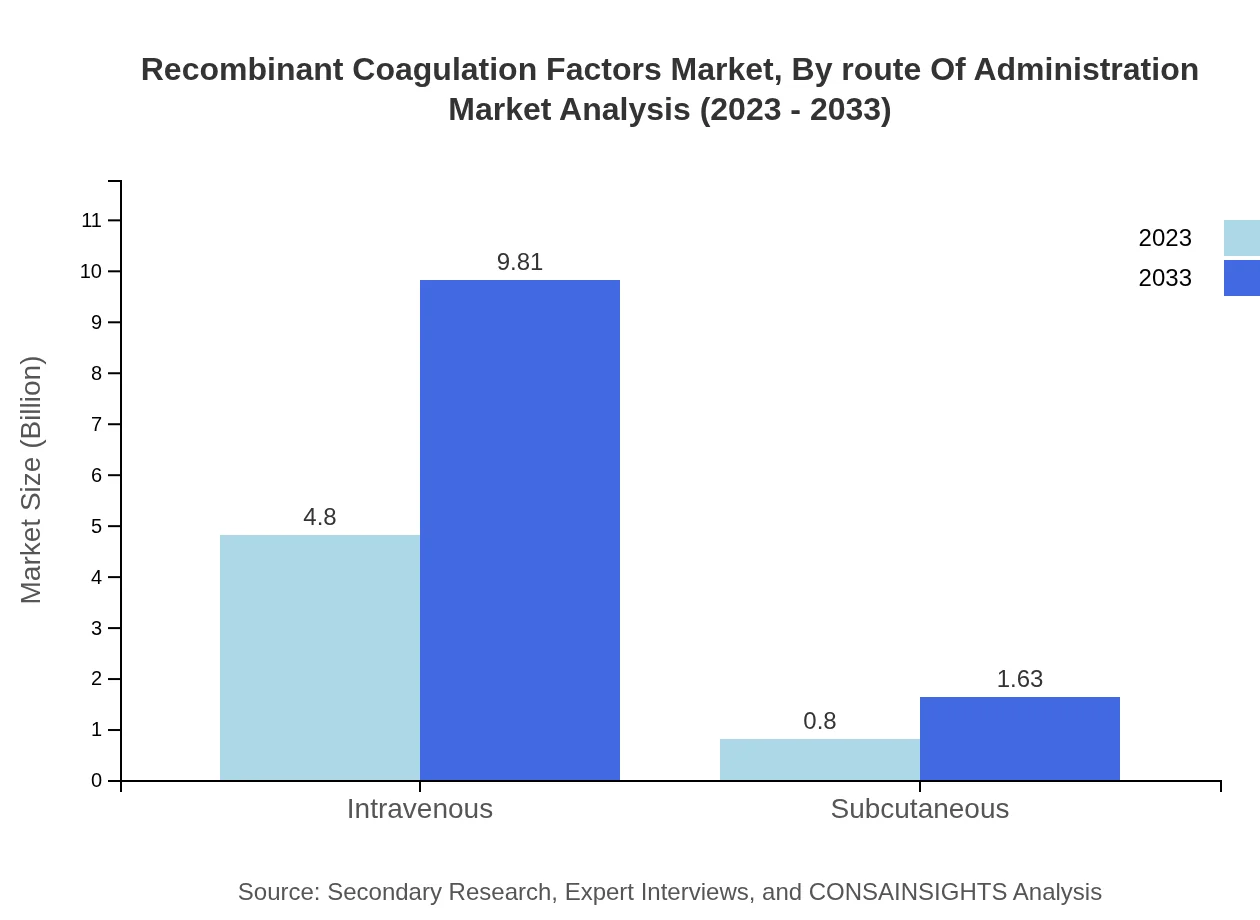
Recombinant Coagulation Factors Market Analysis By Route Of Administration
Intravenous administration represents the bulk of the market share, with a value of $4.80 billion in 2023, growing to $9.81 billion by 2033. Subcutaneous routes, while less common, show growth potential, moving from $0.80 billion to $1.63 billion over the same period.
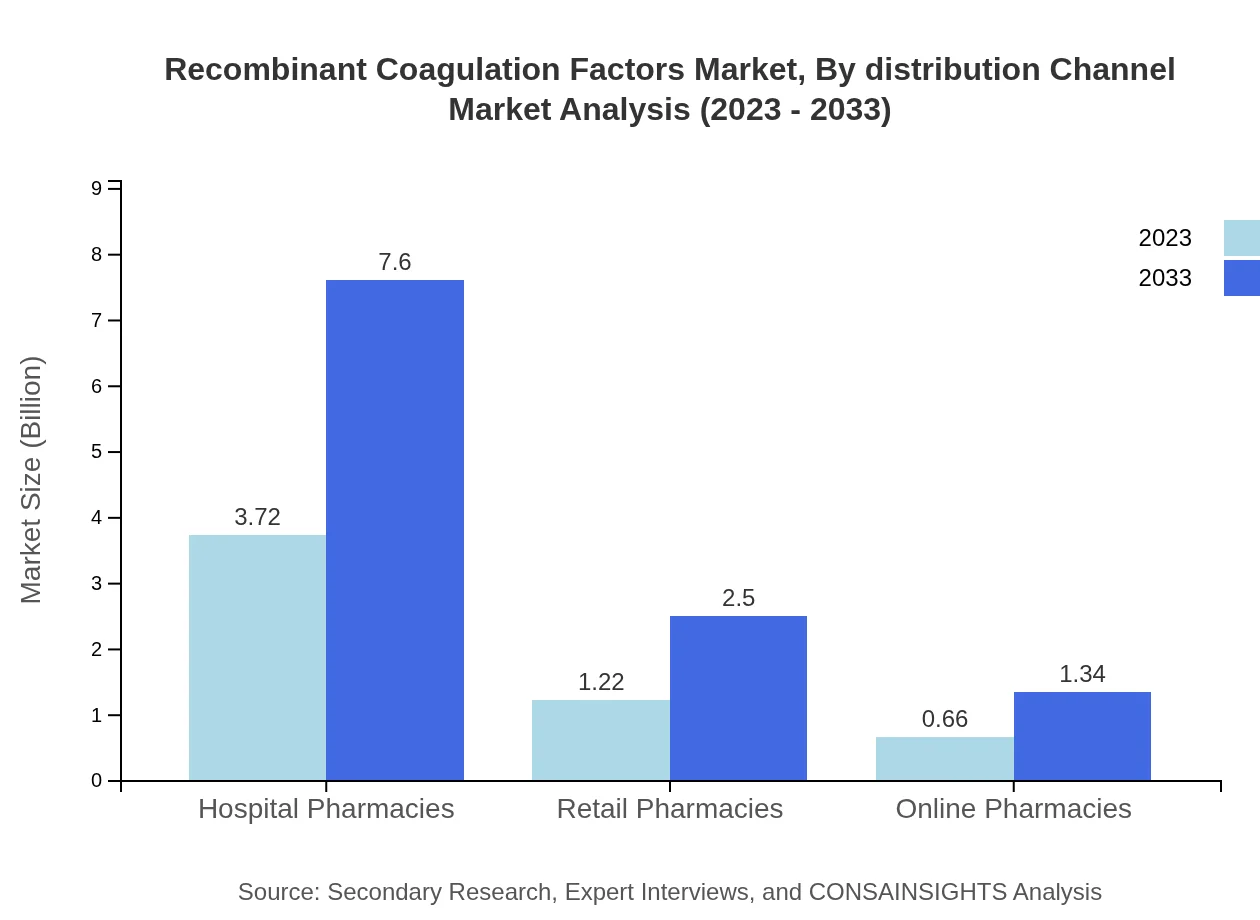
Recombinant Coagulation Factors Market Analysis By Distribution Channel
Hospital pharmacies dominate the distribution channel, valued at $3.72 billion in 2023, set to grow to $7.60 billion by 2033. Retail pharmacies and online pharmacies are also growing, starting from $1.22 billion to $2.50 billion and $0.66 billion to $1.34 billion respectively.
Recombinant Coagulation Factors Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Recombinant Coagulation Factors Industry
Baxter International Inc.:
Baxter is a leading player renowned for its innovative therapies in hemophilia management, producing a range of recombinant coagulation factors that focus on improved patient outcomes.CSL Behring:
CSL Behring is a global leader in clotting factor products, committed to providing high-quality recombinant therapies and advancing treatment options for patients with bleeding disorders.Boehringer Ingelheim GmbH:
Boehringer Ingelheim is known for its cutting-edge R&D in the field of biopharmaceuticals, offering a range of recombinant factors aimed at addressing the needs of hemophilia patients.Roche:
With a strong portfolio of biotech products, Roche has significantly contributed to the recombinant factors market through continuous innovation and dedication to patient care.We're grateful to work with incredible clients.









FAQs
What is the market size of recombinant Coagulation Factors?
The recombinant coagulation factors market is valued at approximately $5.6 billion in 2023 and is projected to grow at a CAGR of 7.2% up to 2033, highlighting significant potential for expansion in this vital healthcare sector.
What are the key market players or companies in this recombinant Coagulation Factors industry?
Key players in the recombinant coagulation factors market include major pharmaceutical companies known for their innovative therapies and treatment solutions, focusing chiefly on hemophilia-related products, which define a crucial segment of the overall market.
What are the primary factors driving the growth in the recombinant Coagulation Factors industry?
The growth of the recombinant coagulation factors market is driven by increasing awareness of hemophilia treatments, technological advancements in biotechnology, rising prevalence of bleeding disorders, and enhanced healthcare infrastructure in developing regions.
Which region is the fastest Growing in the recombinant Coagulation Factors?
The fastest-growing region for recombinant coagulation factors is Europe, with market growth projected from $1.99 billion in 2023 to $4.07 billion by 2033, alongside notable growth in Asia Pacific from $1.06 billion to $2.16 billion.
Does ConsaInsights provide customized market report data for the recombinant Coagulation Factors industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs and queries about the recombinant coagulation factors industry, ensuring clients receive relevant insights and actionable information according to their strategic objectives.
What deliverables can I expect from this recombinant Coagulation Factors market research project?
Deliverables from this research project include comprehensive market analysis reports, segmentation data, forecasts, competitive landscape insights, and strategic recommendations to assist stakeholders in making informed decisions in the recombinant coagulation factors market.
What are the market trends of recombinant Coagulation Factors?
Current trends include a shift towards personalized medicine, increased investment in R&D for novel therapies, the rise of online pharmacies, and greater emphasis on preventive treatments, reflecting changing patient needs in the recombinant coagulation factors market.