Recombinant Hormone Market Report
Published Date: 31 January 2026 | Report Code: recombinant-hormone
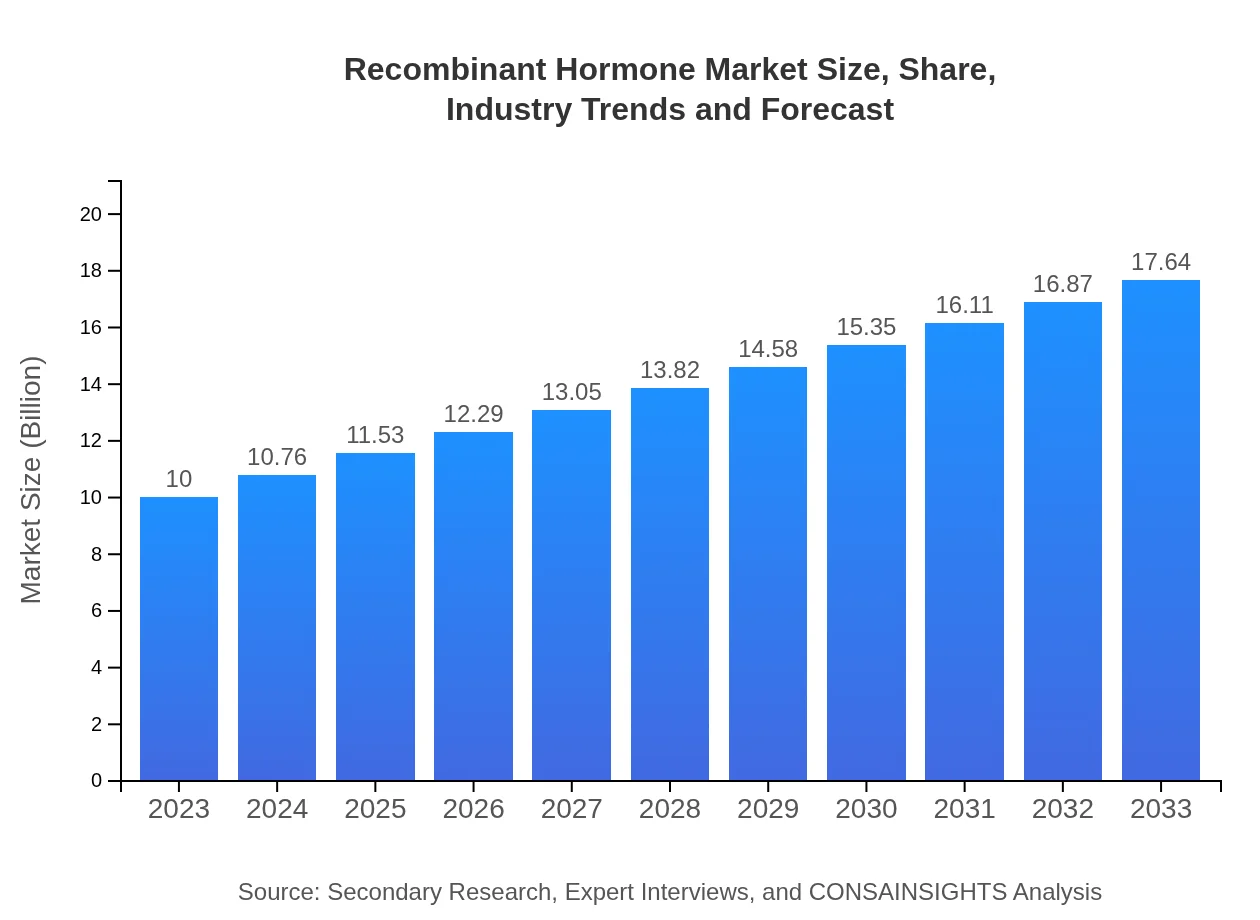
Recombinant Hormone Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the recombinant hormone market, covering market size, growth forecasts from 2023 to 2033, and insights into industry dynamics, regional trends, segmentation, and key players.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $10.00 Billion |
| CAGR (2023-2033) | 5.7% |
| 2033 Market Size | $17.64 Billion |
| Top Companies | Novo Nordisk, Eli Lilly and Company, Boehringer Ingelheim, Genentech |
| Last Modified Date | 31 January 2026 |
Recombinant Hormone Market Overview
Customize Recombinant Hormone Market Report market research report
- ✔ Get in-depth analysis of Recombinant Hormone market size, growth, and forecasts.
- ✔ Understand Recombinant Hormone's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Recombinant Hormone
What is the Market Size & CAGR of Recombinant Hormone market in 2023 and 2033?
Recombinant Hormone Industry Analysis
Recombinant Hormone Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Recombinant Hormone Market Analysis Report by Region
Europe Recombinant Hormone Market Report:
Europe's recombinant hormone market, valued at $3.51 billion in 2023, is expected to reach $6.19 billion by 2033. The region benefits from advanced healthcare systems, regulatory support, and increasing R&D investments in biotechnology.Asia Pacific Recombinant Hormone Market Report:
The Asia Pacific region is expected to witness substantial growth, with the market estimated at $3.35 billion by 2033, up from $1.90 billion in 2023. Factors driving growth include increasing healthcare investments, rising awareness of hormone therapies, and improvements in healthcare infrastructure.North America Recombinant Hormone Market Report:
North America's market is forecasted to increase from $3.21 billion in 2023 to $5.66 billion by 2033, driven by a well-established healthcare system, high prevalence of lifestyle-related diseases, and major pharmaceutical companies focusing on innovative treatments.South America Recombinant Hormone Market Report:
In South America, the recombinant hormone market is projected to grow from $0.95 billion in 2023 to $1.67 billion in 2033. This growth is supported by population growth, urbanization, and rising healthcare access, although economic fluctuations may pose challenges.Middle East & Africa Recombinant Hormone Market Report:
The Middle East and Africa region is expected to see modest growth, with the market anticipated to rise from $0.44 billion in 2023 to $0.77 billion by 2033. Limited healthcare access in some areas remains a significant barrier, although increasing economic development could facilitate market expansion.Tell us your focus area and get a customized research report.
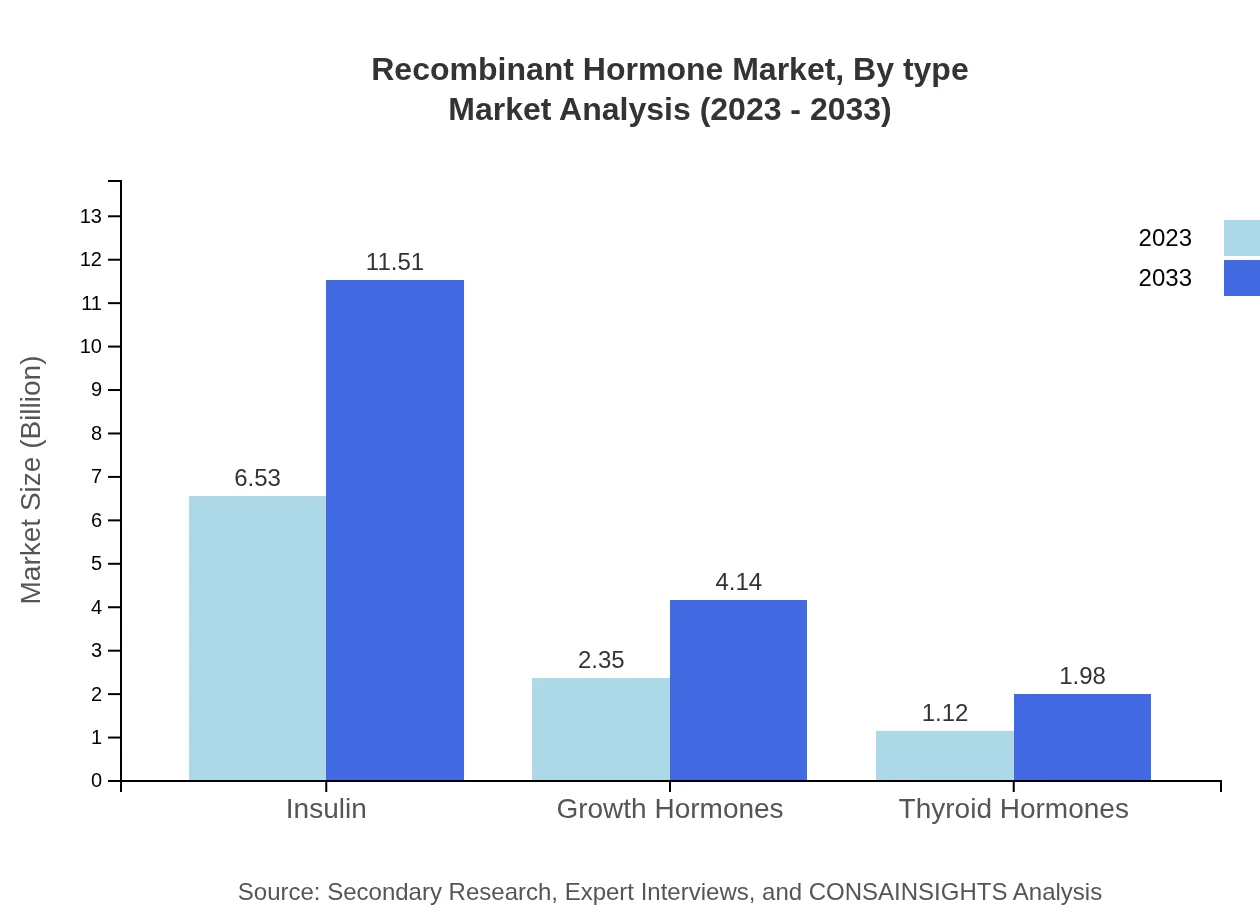
Recombinant Hormone Market Analysis By Type
In terms of product types, Insulin remains the leading segment with a market size of $6.53 billion in 2023, expected to grow to $11.51 billion in 2033, maintaining a market share of 65.27%. Growth hormones follow, anticipated to reach $4.14 billion by 2033 from $2.35 billion in 2023 with a market share of 23.5%. Thyroid hormones, while smaller at $1.12 billion in 2023, will also see growth to reach $1.98 billion by 2033, holding 11.23% market share.
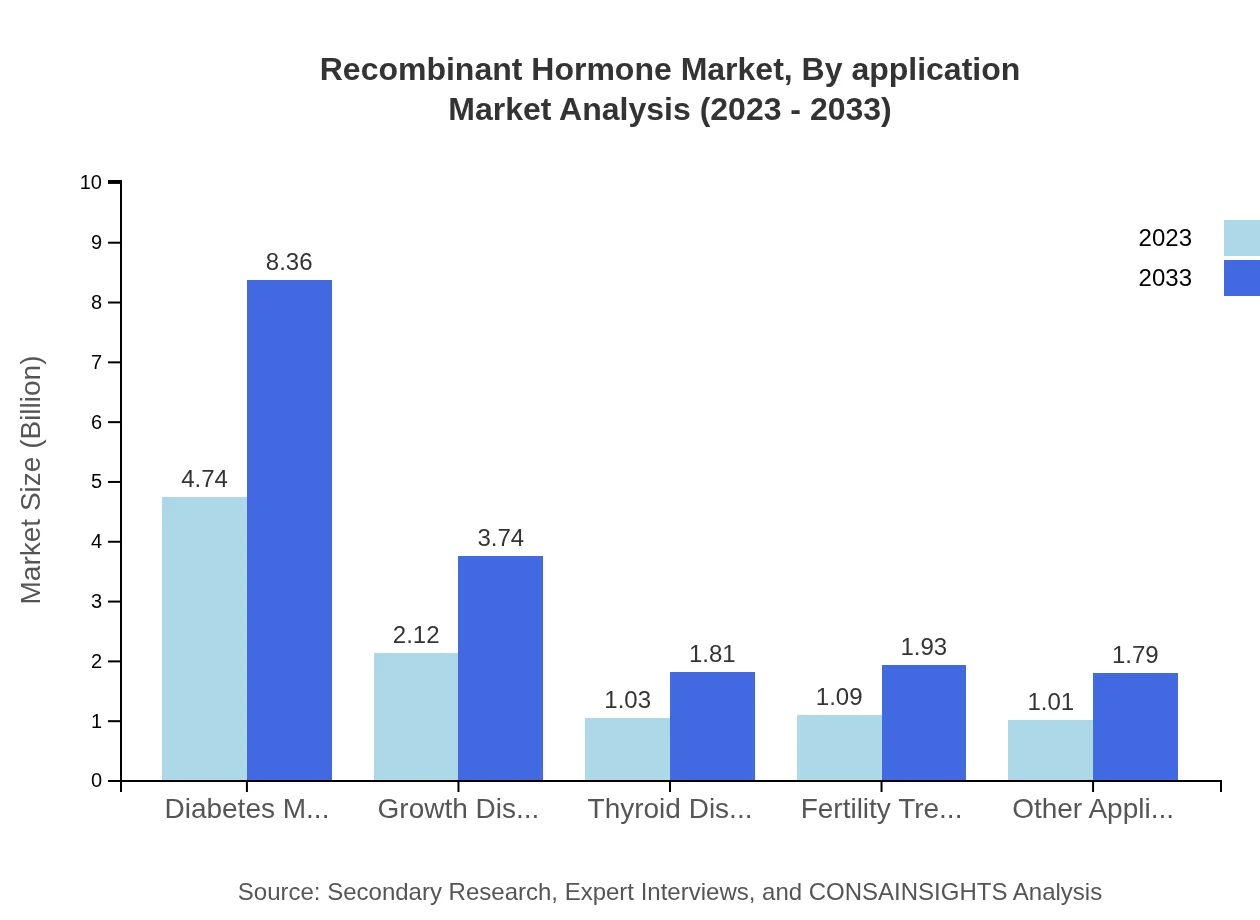
Recombinant Hormone Market Analysis By Application
In terms of application segments, diabetes management commands the largest share at $4.74 billion in 2023, projected to grow to $8.36 billion by 2033. Growth disorders represent a significant sector too, growing from $2.12 billion in 2023 to $3.74 billion in 2033. Thyroid and fertility treatments both present niche opportunities with expected growth in demand driven by rising incidence of related disorders.
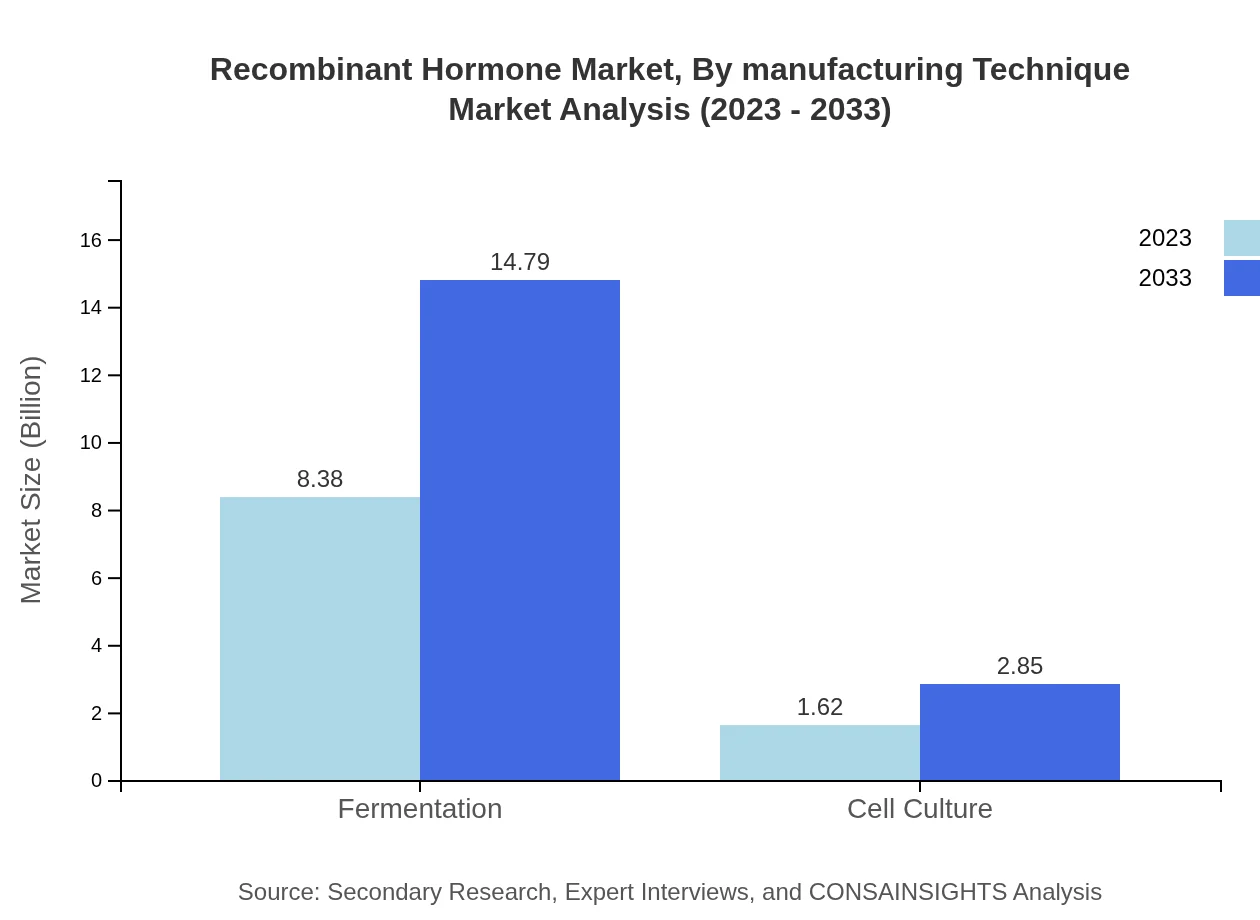
Recombinant Hormone Market Analysis By Manufacturing Technique
The market is dominated by fermentation methods, which currently represent $8.38 billion in sales for 2023 and are set to grow to $14.79 billion by 2033, comprising 83.84% market share. Cell culture techniques, while smaller, are projected to grow steadily from $1.62 billion in 2023 to $2.85 billion in 2033, representing a market share of 16.16%.
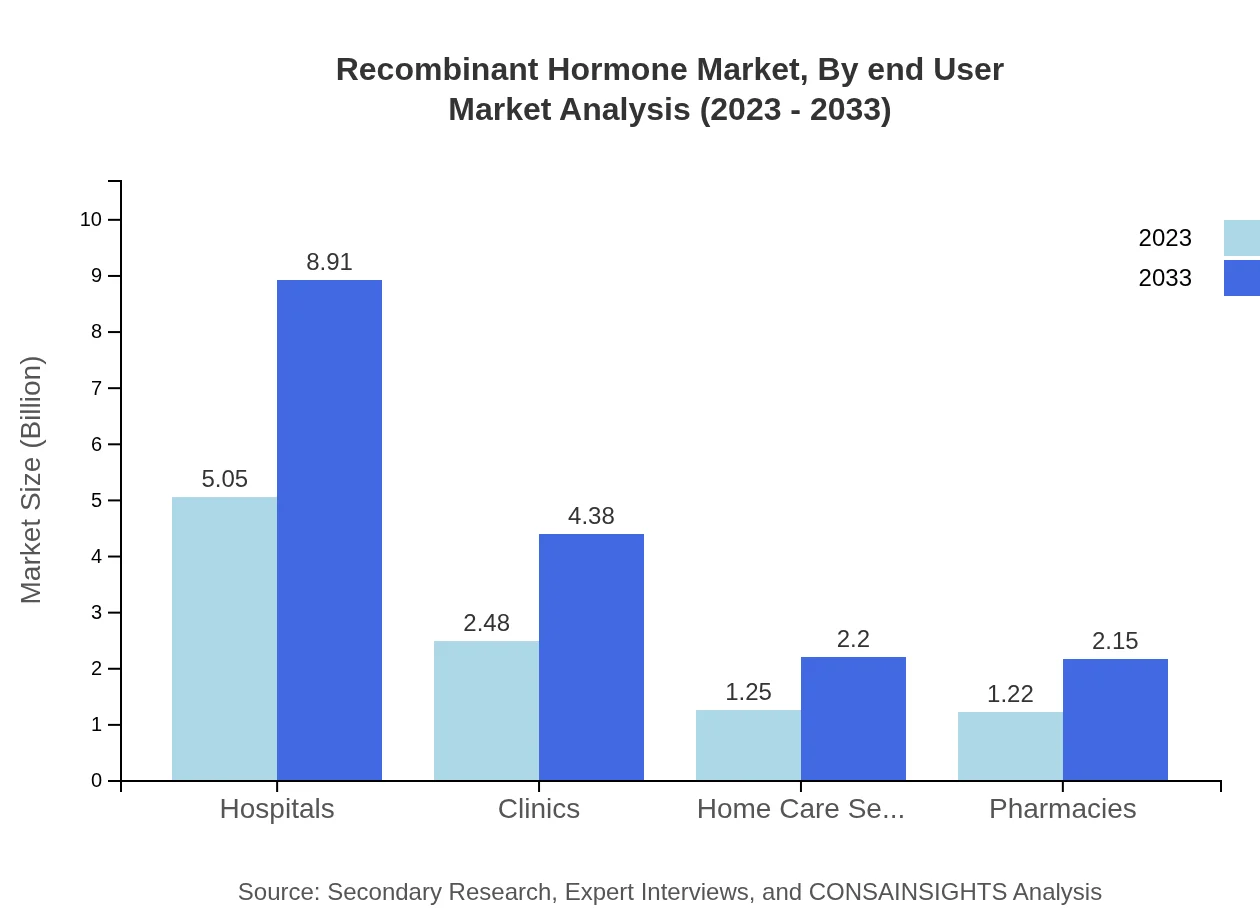
Recombinant Hormone Market Analysis By End User
Hospitals are the primary end users, representing a significant part of the market at $5.05 billion in 2023, expected to increase to $8.91 billion by 2033, capturing a market share of 50.52%. Clinics also show some presence at $2.48 billion, expected to reach $4.38 billion by 2033. Home care settings and pharmacies contribute smaller yet notable shares to the overall market.
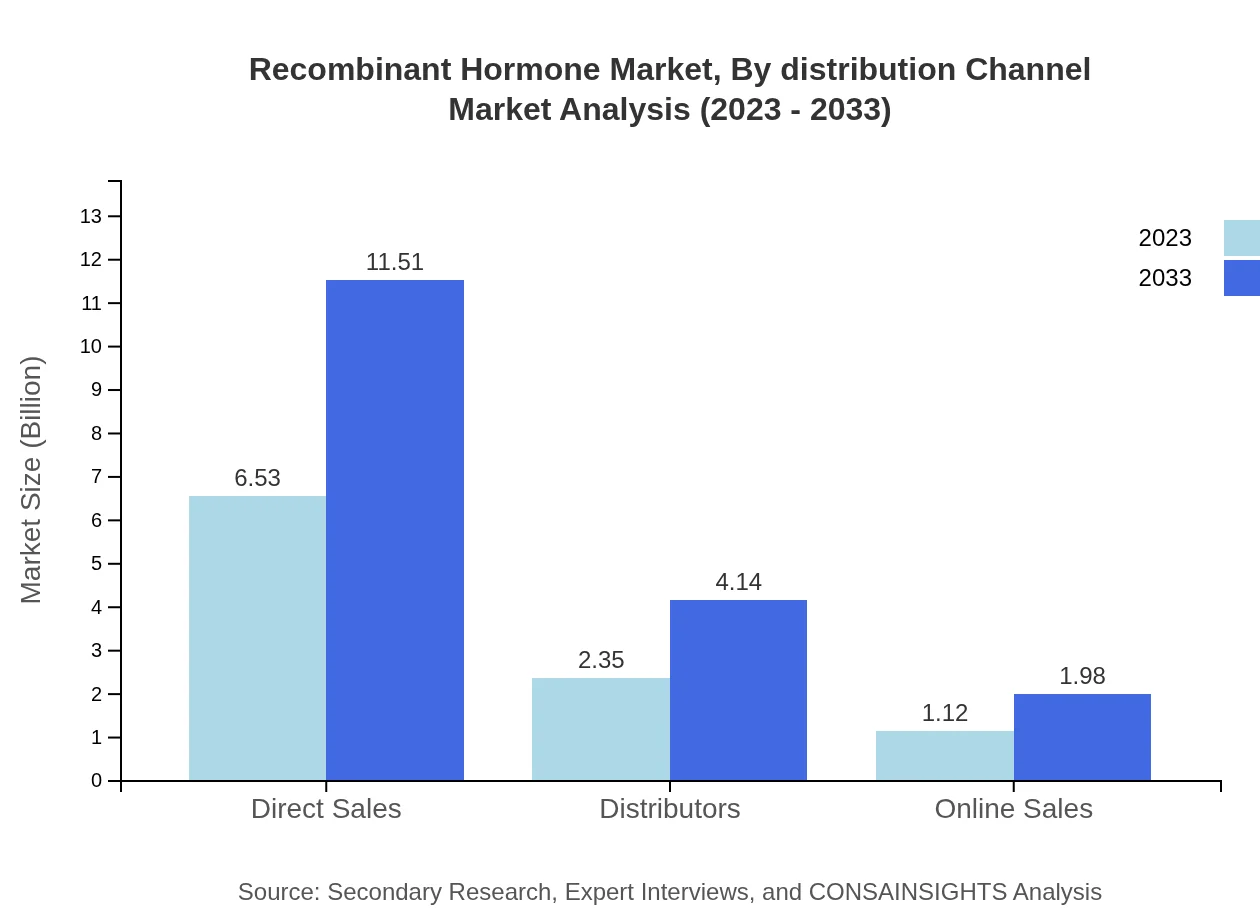
Recombinant Hormone Market Analysis By Distribution Channel
Direct sales dominate the distribution channels, with approximately $6.53 billion in 2023 and a further growth anticipated to $11.51 billion by 2033, holding 65.27% market share. Distributors and online sales also represent significant channels, with projected growth reflecting increased convenience in purchasing strategies.
Recombinant Hormone Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Recombinant Hormone Industry
Novo Nordisk:
A leader in diabetes care, Novo Nordisk is one of the largest producers of insulin globally, focusing on innovative diabetes solutions and hormone therapies.Eli Lilly and Company:
Known for its extensive portfolio of diabetes medications, including insulin products, Eli Lilly is at the forefront of recombinant hormone development and manufacturing.Boehringer Ingelheim:
A significant player in the biopharmaceutical sector, Boehringer Ingelheim invests heavily in recombinant proteins and hormones, including insulin and growth hormones.Genentech:
As a pioneer in biotechnology, Genentech focuses on innovative treatment solutions, including recombinant hormones for various medical needs.We're grateful to work with incredible clients.









FAQs
What is the market size of recombinant Hormone?
The recombinant hormone market is currently valued at approximately $10 billion and is expected to grow at a CAGR of 5.7% from 2023 to 2033, driven by advancements in biotechnology and increasing healthcare demand.
What are the key market players or companies in the recombinant hormone industry?
Key players in the recombinant hormone market include major biotechnology firms that specialize in hormone production, pharmaceuticals, and healthcare innovations, although specific company names are not disclosed here.
What are the primary factors driving the growth in the recombinant hormone industry?
Growth in the recombinant hormone market is primarily driven by rising incidence of hormonal disorders, advances in biotechnology and drug development, and increasing government investment in healthcare research and innovation.
Which region is the fastest Growing in the recombinant hormone market?
The Asia Pacific region is the fastest-growing market for recombinant hormones, projected to expand from $1.90 billion in 2023 to $3.35 billion by 2033, reflecting increasing healthcare expenditures and demand for innovative treatments.
Does ConsaInsights provide customized market report data for the recombinant hormone industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the recombinant hormone industry, addressing unique parameters and insights for client requirements.
What deliverables can I expect from this recombinant hormone market research project?
Expect comprehensive deliverables including detailed market analysis, growth forecasts, competitive landscape breakdown, segmentation data, and actionable insights tailored for strategic decision-making.
What are the market trends of recombinant hormone?
Current trends in the recombinant hormone market include increasing focus on personalized medicine, enhanced production technologies such as fermentation and cell culture, and growth in telemedicine for hormone therapies.