Recombinant Protein Market Report
Published Date: 31 January 2026 | Report Code: recombinant-protein
Recombinant Protein Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Recombinant Protein market from 2023 to 2033, including insights into market size, growth trends, segmentation, regional dynamics, and competitive landscape.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
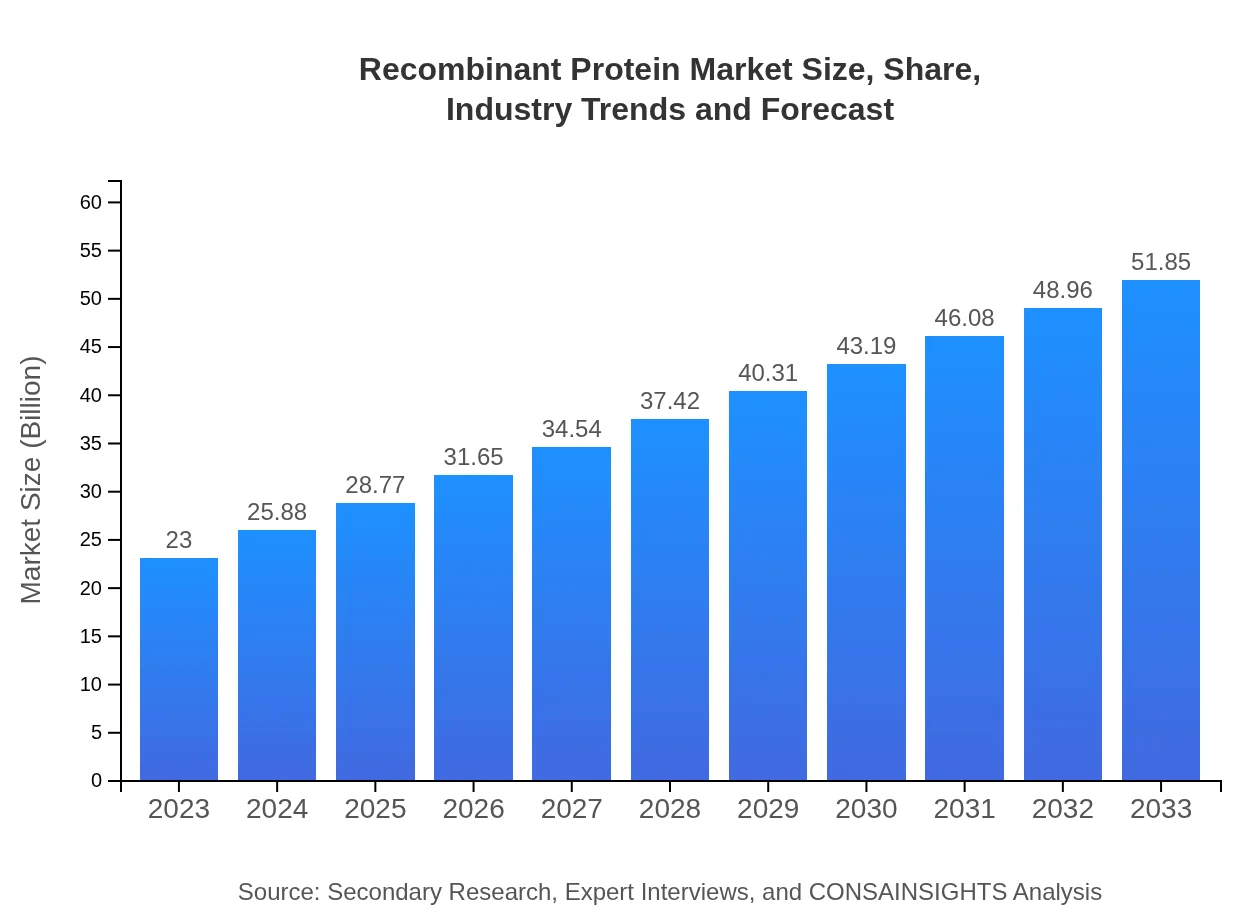
| 2023 Market Size | $23.00 Billion |
| CAGR (2023-2033) | 8.2% |
| 2033 Market Size | $51.85 Billion |
| Top Companies | Amgen Inc., Genentech, Inc., AbbVie Inc. |
| Last Modified Date | 31 January 2026 |
Recombinant Protein Market Overview
Customize Recombinant Protein Market Report market research report
- ✔ Get in-depth analysis of Recombinant Protein market size, growth, and forecasts.
- ✔ Understand Recombinant Protein's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Recombinant Protein
What is the Market Size & CAGR of Recombinant Protein market in 2023?
Recombinant Protein Industry Analysis
Recombinant Protein Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Recombinant Protein Market Analysis Report by Region
Europe Recombinant Protein Market Report:
The European Recombinant Protein market, worth $5.54 billion in 2023, is projected to reach $12.49 billion by 2033. The market benefits from stringent regulatory frameworks and funding initiatives aimed at fostering biopharmaceutical innovations.Asia Pacific Recombinant Protein Market Report:
In 2023, the Recombinant Protein market in the Asia Pacific is valued at $4.62 billion and is expected to reach $10.42 billion by 2033, demonstrating a significant growth trajectory. This expansion is supported by increasing investments in biotechnology and healthcare infrastructure, along with a rising incidence of chronic diseases in the region.North America Recombinant Protein Market Report:
The North American market is the largest, estimated at $8.22 billion in 2023 and expected to grow to $18.52 billion by 2033. This growth is driven by robust R&D expenditures, a strong presence of key market players, and advanced healthcare systems.South America Recombinant Protein Market Report:
The South American market, valued at $1.76 billion in 2023, is projected to increase to $3.96 billion by 2033. Factors contributing to this growth include improvements in healthcare access and rising awareness about advanced therapeutic options.Middle East & Africa Recombinant Protein Market Report:
In the Middle East and Africa, the market was $2.86 billion in 2023 and is anticipated to grow to $6.45 billion by 2033. This growth is stimulated by improvements in healthcare quality and increased investment in biopharmaceutical manufacturing.Tell us your focus area and get a customized research report.
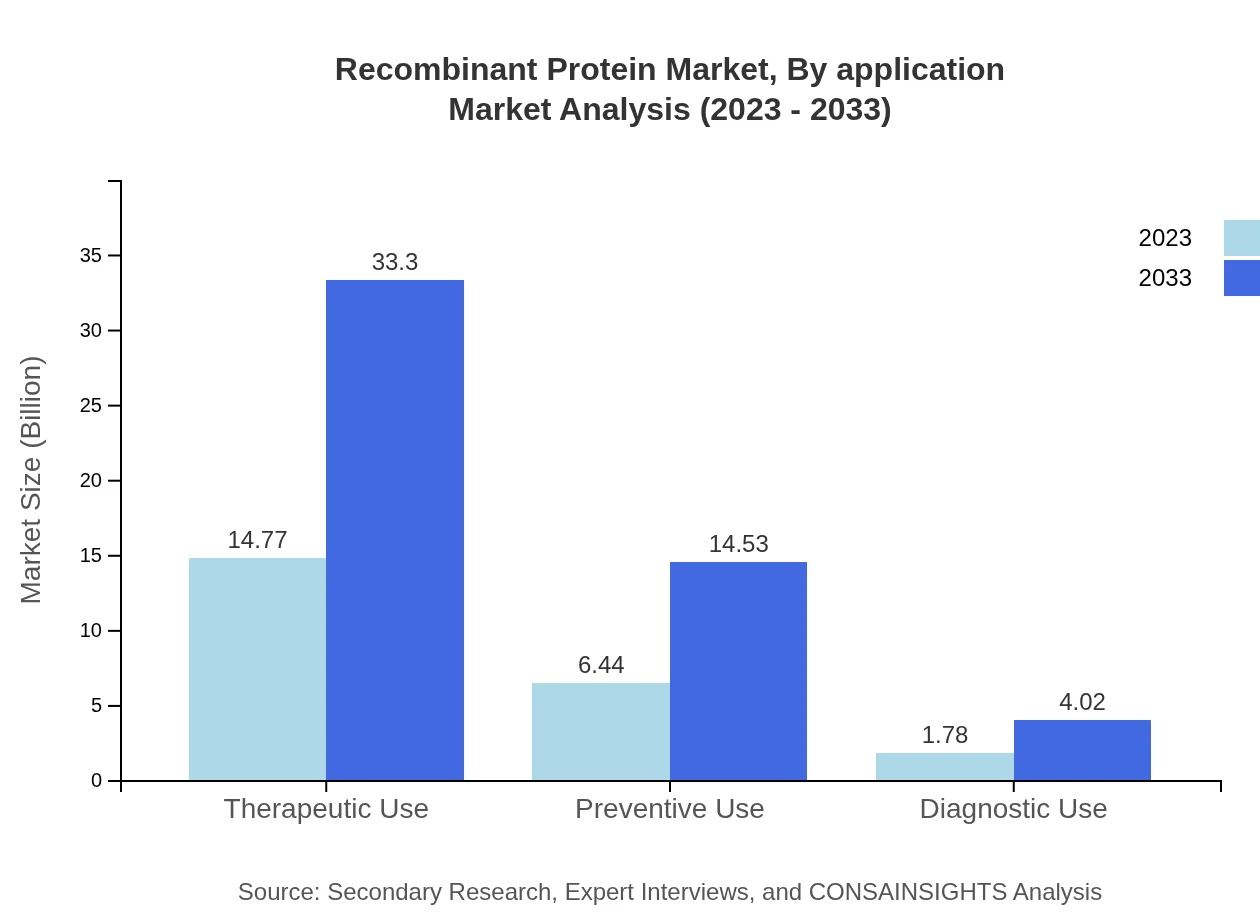
Recombinant Protein Market Analysis By Application
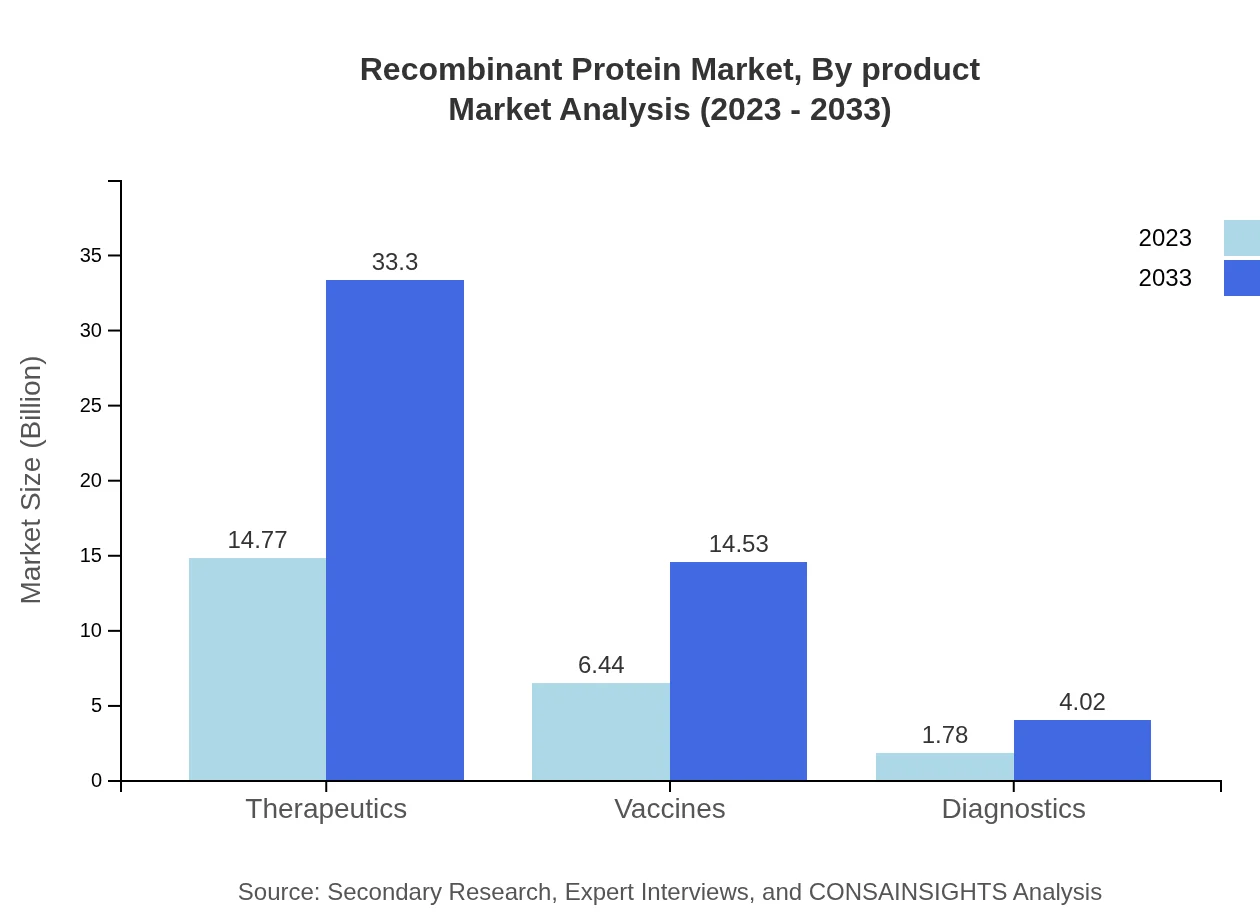
The Recombinant Protein market by application is dominated by therapeutics, which is valued at $14.77 billion in 2023 and projected to grow to $33.30 billion by 2033. Other notable applications include vaccines ($6.44 billion in 2023 to $14.53 billion in 2033) and diagnostics ($1.78 billion in 2023 to $4.02 billion in 2033), reflecting the increasing reliance on biologics in various medical fields.
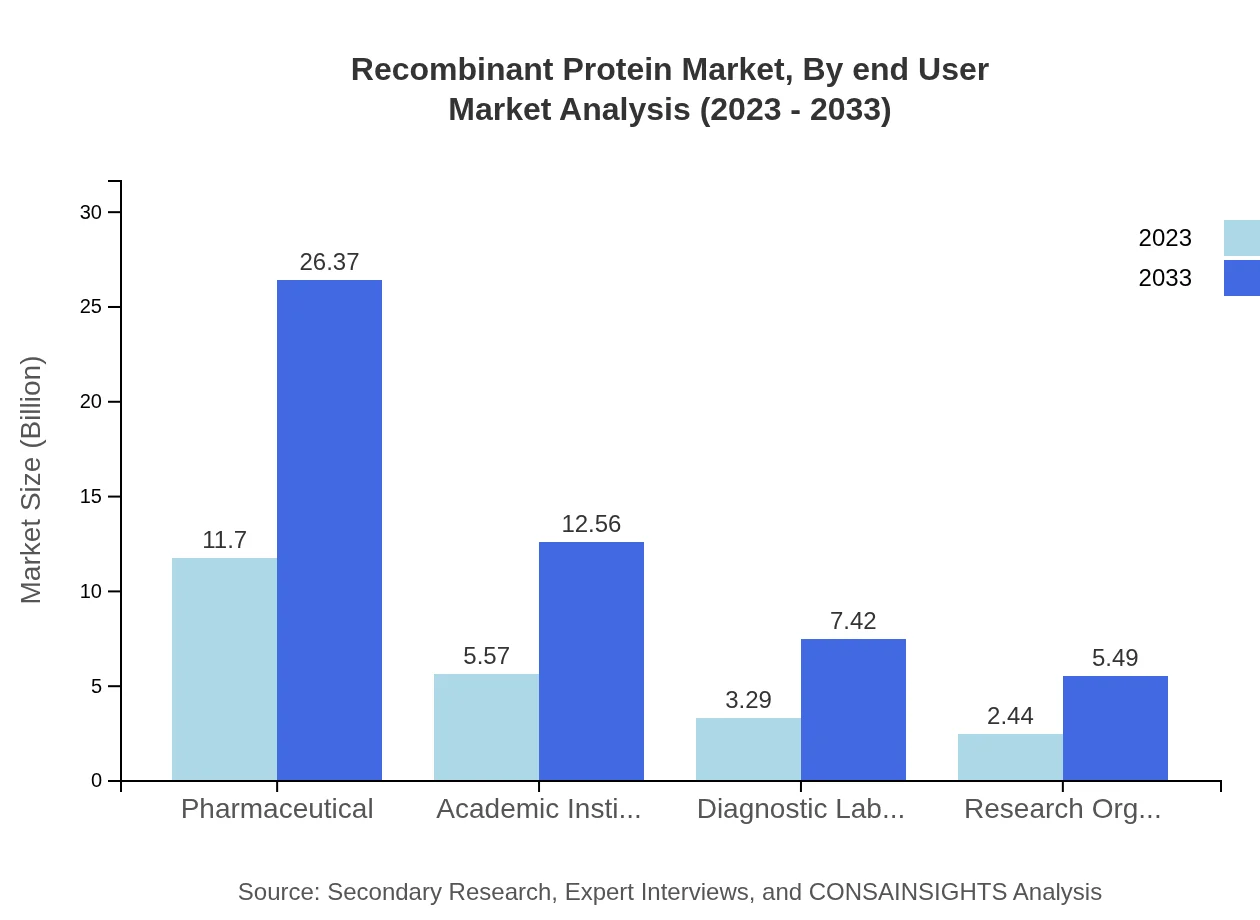
Recombinant Protein Market Analysis By End User
The primary end-user for Recombinant Proteins is the pharmaceutical segment, which holds a market share of approximately 50.87% in 2023 and is expected to maintain a similar share through 2033. Academic institutions also represent a significant share, poised to grow due to increasing research initiatives.
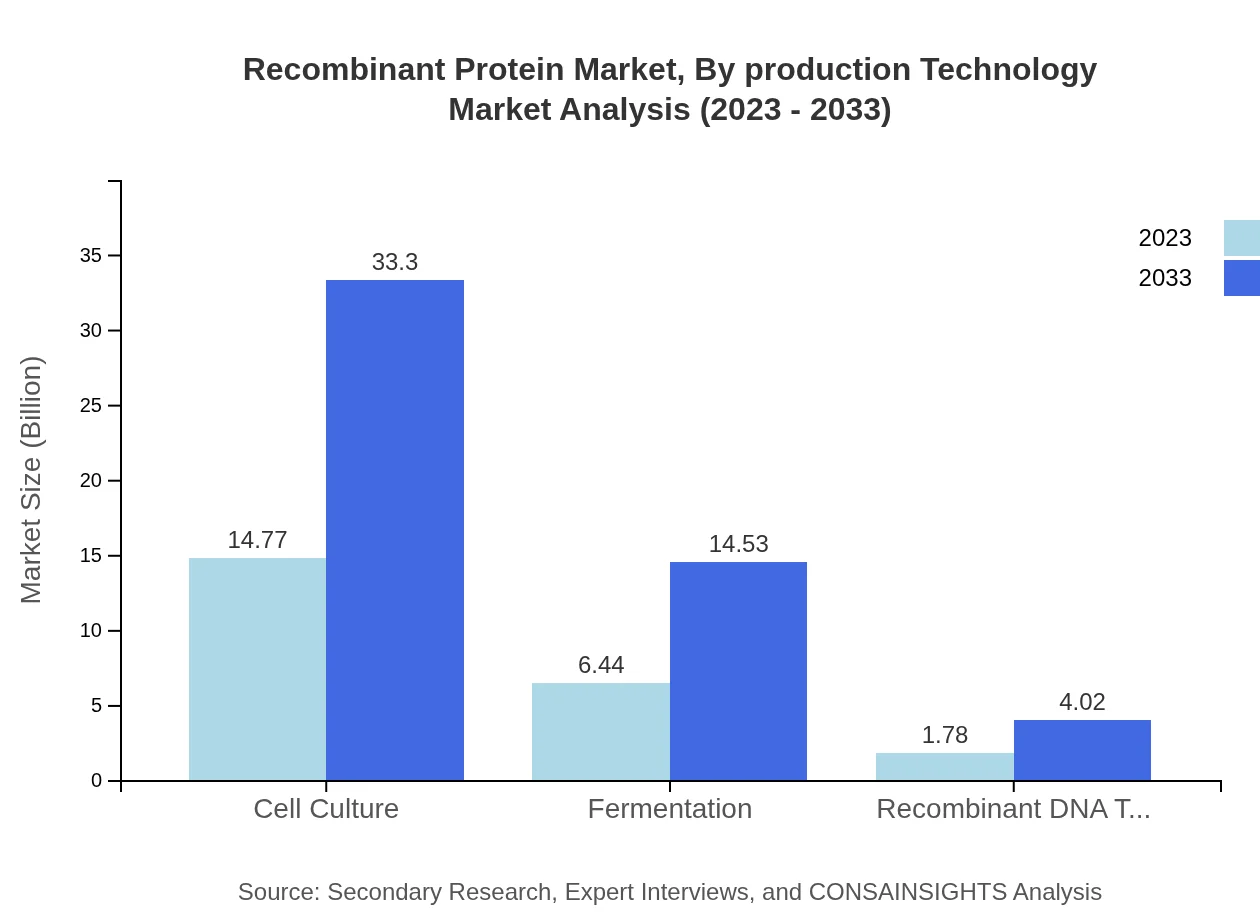
Recombinant Protein Market Analysis By Production Technology
Cell culture-based production accounted for a notable share, with values projected to increase from $14.77 billion in 2023 to $33.30 billion by 2033. Fermentation technology remains prevalent, growing from $6.44 billion to $14.53 billion in the same period, driven by its efficacy in large-scale protein production.
Recombinant Protein Market Analysis By Product
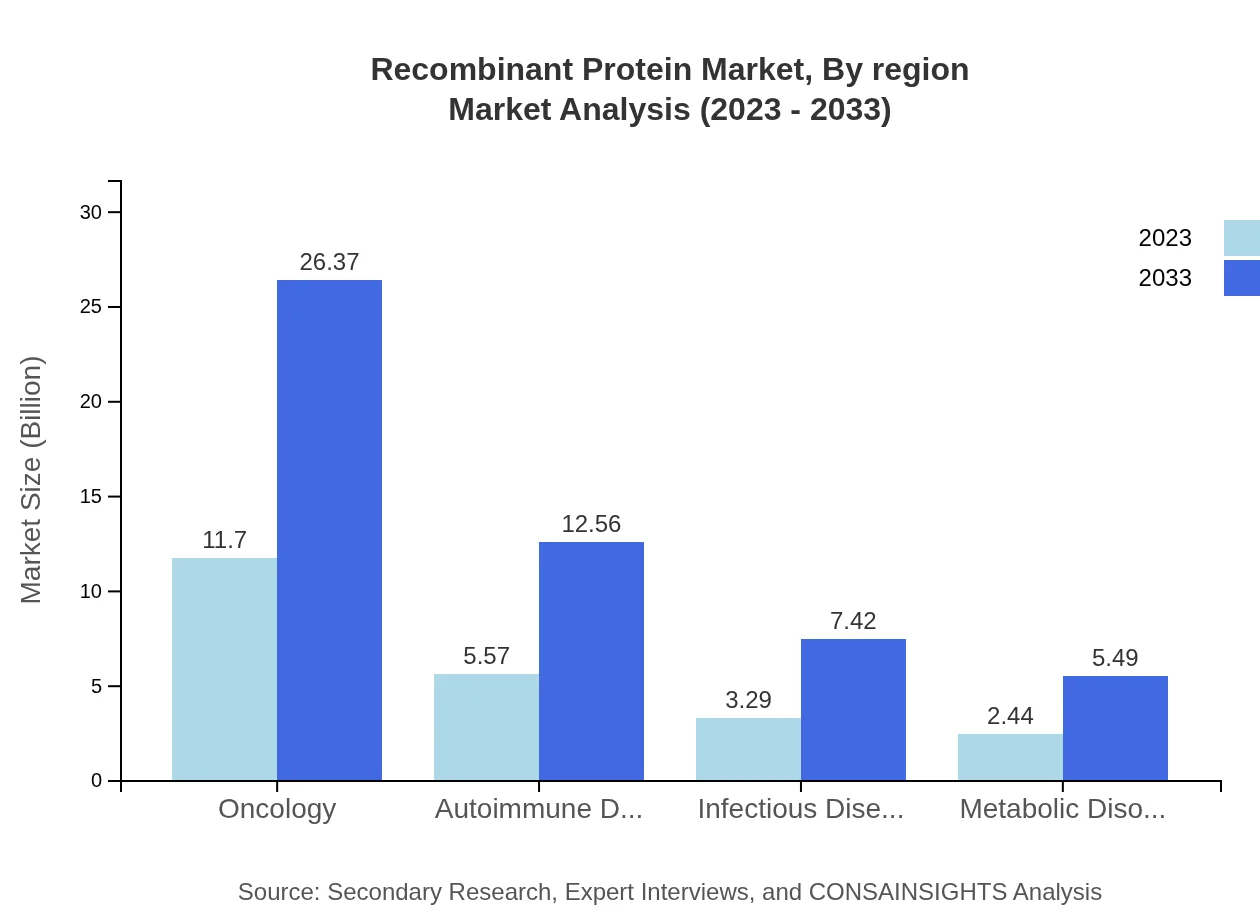
Therapeutic proteins dominate the market, with sizes of $11.70 billion in 2023 to $26.37 billion by 2033. Vaccines also show significant growth potential, reflecting a rising focus on preventive healthcare.
Recombinant Protein Market Analysis By Region
Oncology is a leading therapeutic segment, expanding from $11.70 billion in 2023 to $26.37 billion in 2033, sustained by ongoing treatments and drug developments. Autoimmune disorders and infectious diseases also hold significant shares, with strong growth projected driven by increasing disease prevalence.
Recombinant Protein Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Recombinant Protein Industry
Amgen Inc.:
A pioneer in biotechnology, Amgen focuses on developing therapies for cancer and other serious diseases using recombinant protein technologies.Genentech, Inc.:
Part of the Roche Group, Genentech is renowned for its innovative biopharmaceuticals, including monoclonal antibodies and other recombinant proteins.AbbVie Inc.:
AbbVie specializes in therapies derived from recombinant technologies, notably in immunology and oncology, demonstrating significant innovation in drug development.We're grateful to work with incredible clients.









FAQs
What is the market size of recombinant Protein?
The global recombinant protein market is projected to reach approximately $23 billion in 2023, with a compound annual growth rate (CAGR) of 8.2% expected to sustain through 2033.
What are the key market players or companies in the recombinant Protein industry?
Key players in the recombinant protein industry include prominent biopharmaceutical companies such as Amgen, Genentech, and Eli Lilly, leading the market with innovative products and extensive research capabilities.
What are the primary factors driving the growth in the recombinant Protein industry?
Growth factors include increasing prevalence of chronic diseases, advancements in biotechnology, and rising investments in R&D of therapeutic proteins and vaccines, all contributing to the expanding market landscape.
Which region is the fastest Growing in the recombinant Protein market?
North America is identified as the fastest-growing region, with market size escalating from $8.22 billion in 2023 to approximately $18.52 billion by 2033, driven by robust healthcare infrastructure and innovation.
Does ConsaInsights provide customized market report data for the recombinant Protein industry?
Yes, ConsaInsights offers tailored market report data to fit specific client needs in the recombinant protein industry, ensuring relevant insights for decision-making and strategic planning.
What deliverables can I expect from this recombinant Protein market research project?
Expect comprehensive reports featuring market size analysis, growth forecasts, competitive landscape insights, and strategic recommendations, all aimed at enhancing informed business decisions in the recombinant protein sector.
What are the market trends of recombinant Protein?
Current trends include growth in oncology therapeutics, increased focus on personalized medicine, and rising adoption of recombinant proteins in vaccine development, shaping the future of healthcare.