Regulatory Affairs Outsourcing Market Report
Published Date: 31 January 2026 | Report Code: regulatory-affairs-outsourcing
Regulatory Affairs Outsourcing Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Regulatory Affairs Outsourcing market, covering market size, growth trends, segmentation, regional insights, and forecasts from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
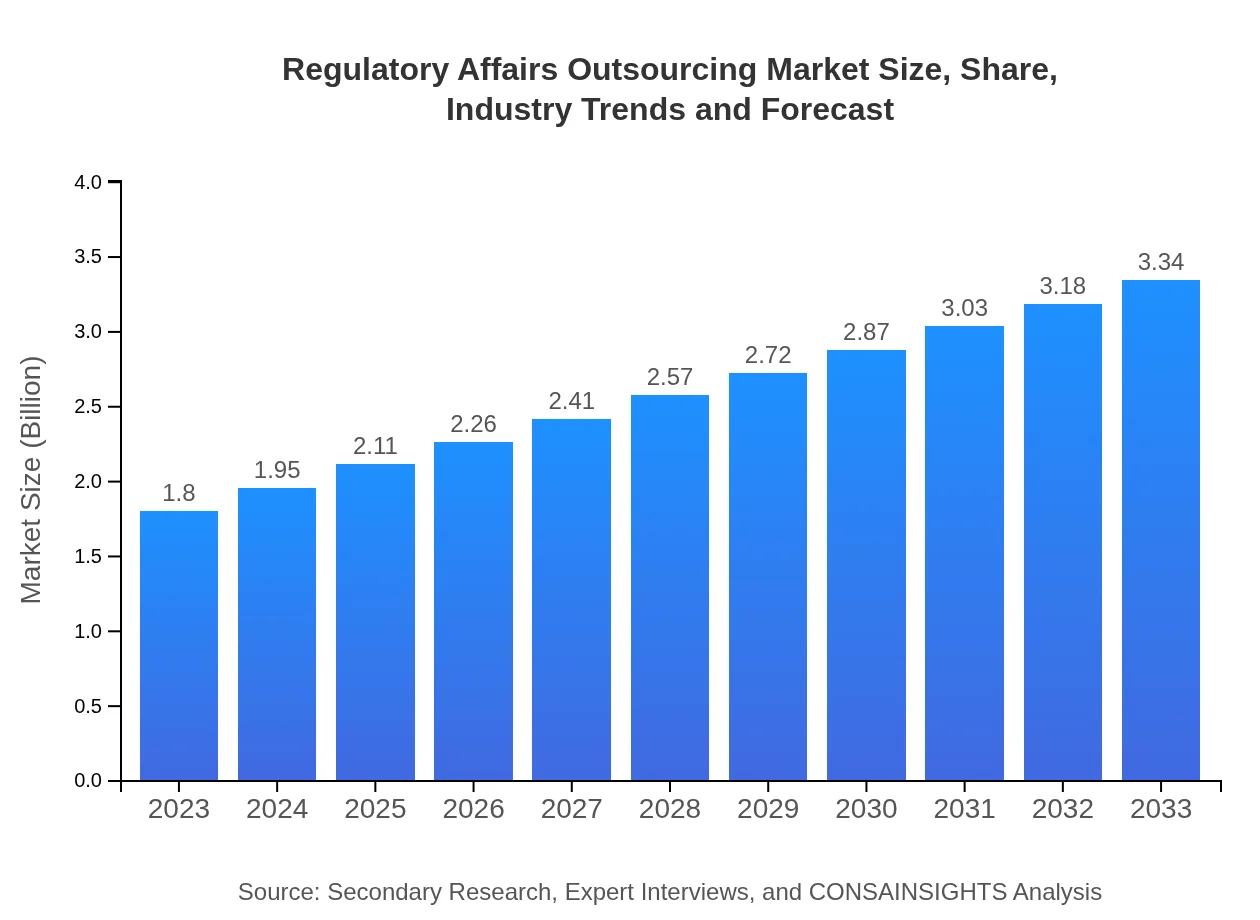
| 2023 Market Size | $1.80 Billion |
| CAGR (2023-2033) | 6.2% |
| 2033 Market Size | $3.34 Billion |
| Top Companies | Covance, PAREXEL, ICON plc, Charles River Laboratories, Regulatory Solutions |
| Last Modified Date | 31 January 2026 |
Regulatory Affairs Outsourcing Market Overview
Customize Regulatory Affairs Outsourcing Market Report market research report
- ✔ Get in-depth analysis of Regulatory Affairs Outsourcing market size, growth, and forecasts.
- ✔ Understand Regulatory Affairs Outsourcing's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Regulatory Affairs Outsourcing
What is the Market Size & CAGR of Regulatory Affairs Outsourcing market in 2033?
Regulatory Affairs Outsourcing Industry Analysis
Regulatory Affairs Outsourcing Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Regulatory Affairs Outsourcing Market Analysis Report by Region
Europe Regulatory Affairs Outsourcing Market Report:
In Europe, the market for Regulatory Affairs Outsourcing was valued at USD 0.53 billion in 2023, predicted to increase to USD 0.98 billion by 2033. The region benefits from strong regulatory frameworks and a high concentration of pharmaceutical companies.Asia Pacific Regulatory Affairs Outsourcing Market Report:
In 2023, the Asia Pacific market for Regulatory Affairs Outsourcing is valued at USD 0.37 billion, projected to grow to USD 0.68 billion by 2033. This growth is primarily attributed to the rapid expansion of the pharmaceutical industry in countries like China and India, coupled with increasing investments in biotechnology and life sciences.North America Regulatory Affairs Outsourcing Market Report:
The North America market is significantly leading with a size of USD 0.58 billion in 2023, anticipated to grow to USD 1.08 billion by 2033. This region benefits from stringent regulatory requirements and the presence of key market players, fostering a more competitive outsourcing environment.South America Regulatory Affairs Outsourcing Market Report:
South America represented a market size of USD 0.17 billion in 2023, expected to reach USD 0.32 billion by 2033. The growth in this region is fueled by rising healthcare needs and the establishment of various offshore R&D centers.Middle East & Africa Regulatory Affairs Outsourcing Market Report:
The Middle East and Africa market stood at USD 0.15 billion in 2023, with forecasts suggesting growth to USD 0.27 billion by 2033. This growth is driven by increasing collaboration between regulatory bodies and local manufacturers to enhance healthcare delivery.Tell us your focus area and get a customized research report.
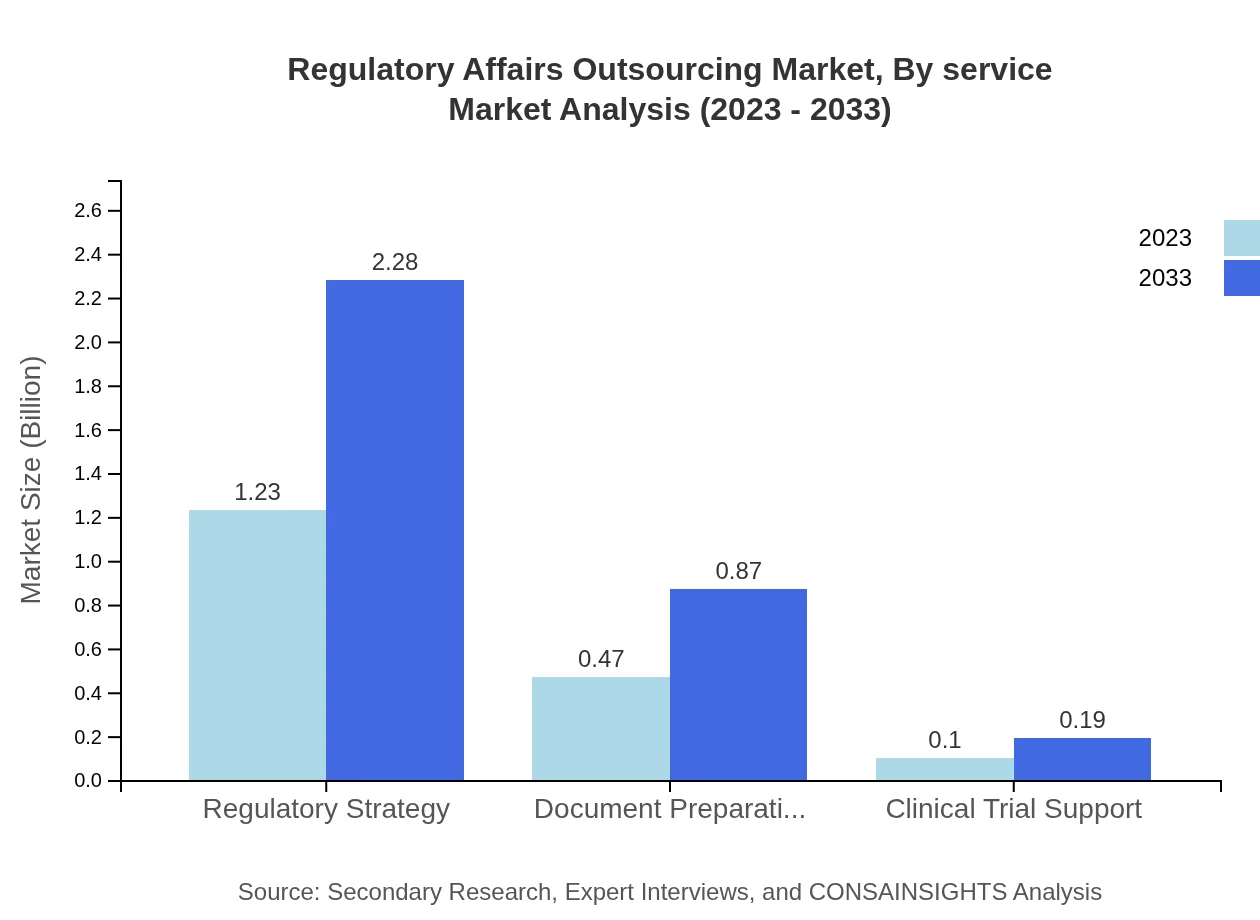
Regulatory Affairs Outsourcing Market Analysis By Service
The Regulatory Affairs Outsourcing market is predominantly segmented into key service types. Regulatory Strategy leads with a market size of USD 1.23 billion in 2023 and expected to grow to USD 2.28 billion by 2033. Document Preparation follows, growing from USD 0.47 billion to USD 0.87 billion in the same period. Clinical Trial Support starts at USD 0.10 billion, growing to USD 0.19 billion.
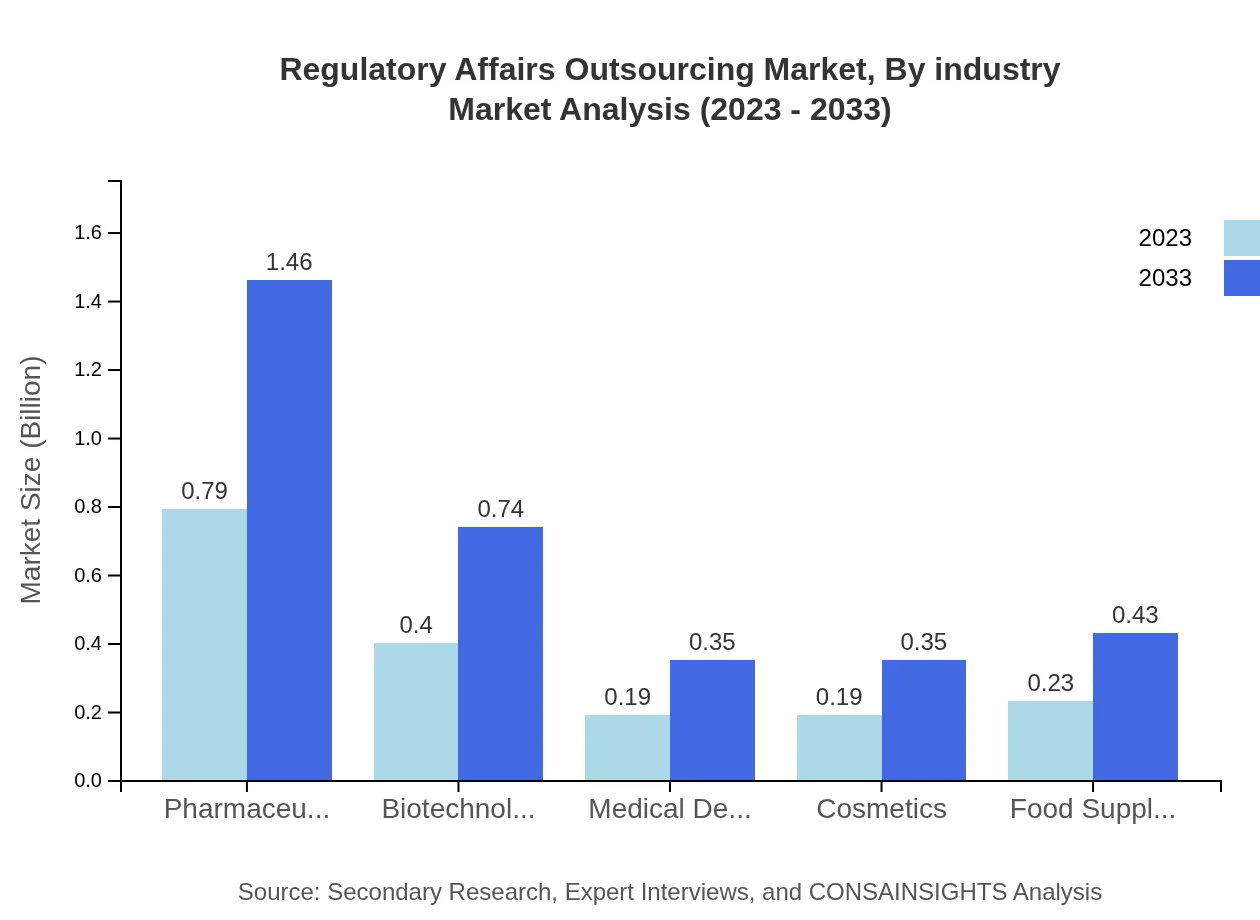
Regulatory Affairs Outsourcing Market Analysis By Industry
Within the industry segmentation, Pharmaceuticals dominate, accounting for USD 0.79 billion in 2023, anticipated to rise to USD 1.46 billion by 2033. The Biotechnology sector shows a significant growth pattern from USD 0.40 billion to USD 0.74 billion. Other segments like Medical Devices, Cosmetics, and Food Supplements are also witnessing notable growth, contributing to 10% - 13% shares.
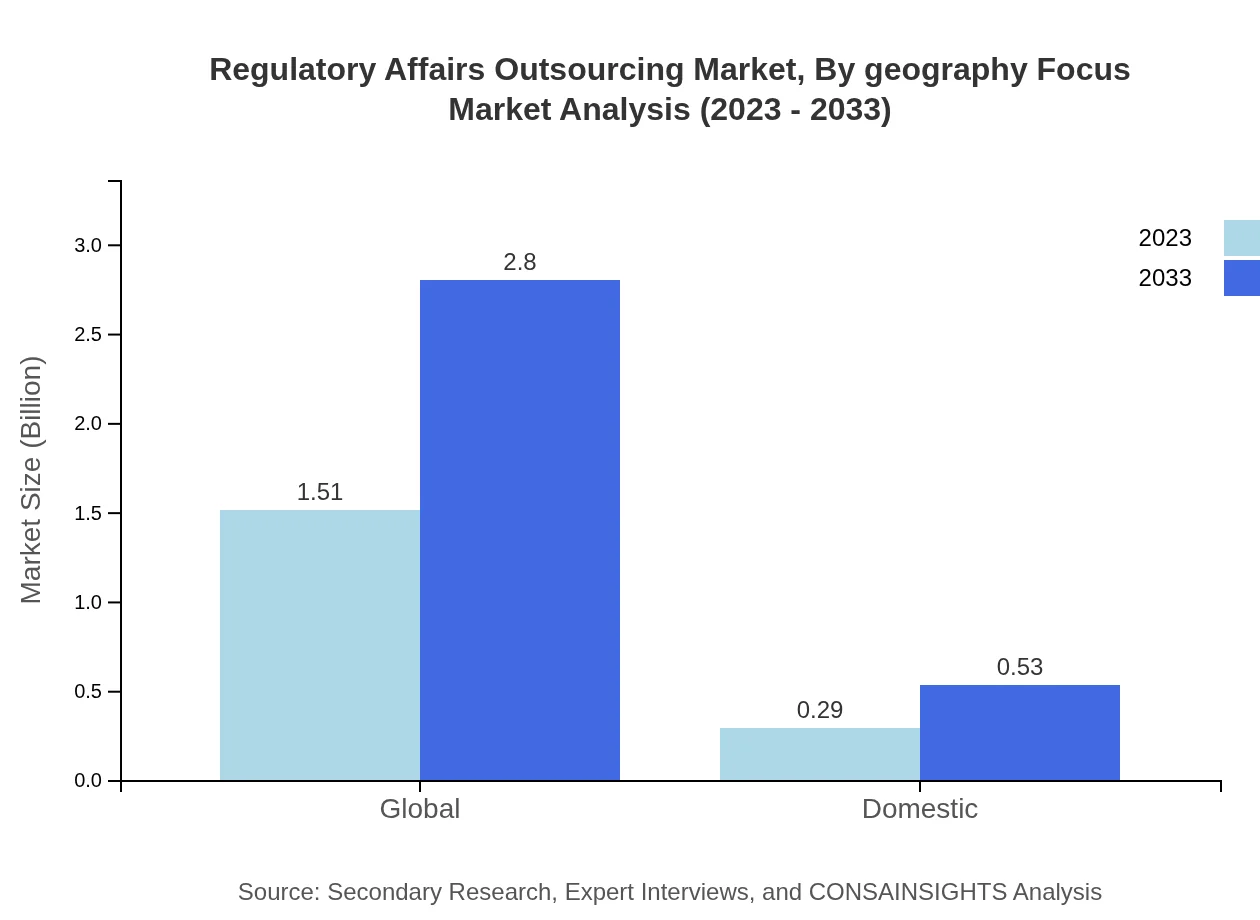
Regulatory Affairs Outsourcing Market Analysis By Geography Focus
Geographically, the market showcases diverse growth patterns. North America holds the largest share with 58%, followed by Europe at 43%. Asia Pacific and South America are emerging as growth hotspots, with Asia expected to double its market value over the next decade.
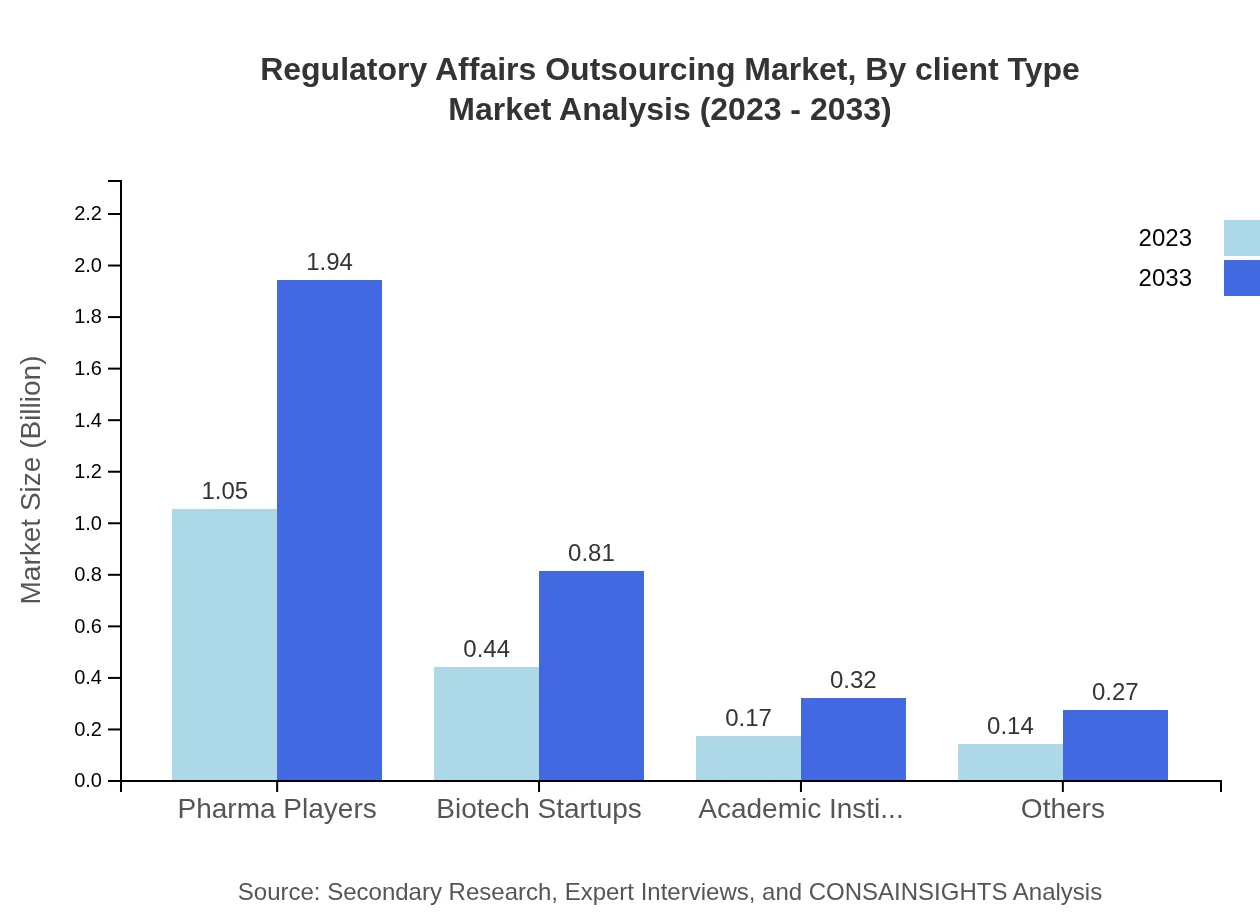
Regulatory Affairs Outsourcing Market Analysis By Client Type
The client type segmentation reveals that Pharma Players are at the forefront, holding 58.17% of the market share as of 2023, while Biotech Startups account for 24.17%. Academic Institutions and others contribute to the remainder, reflecting a diverse client base seeking regulatory support.
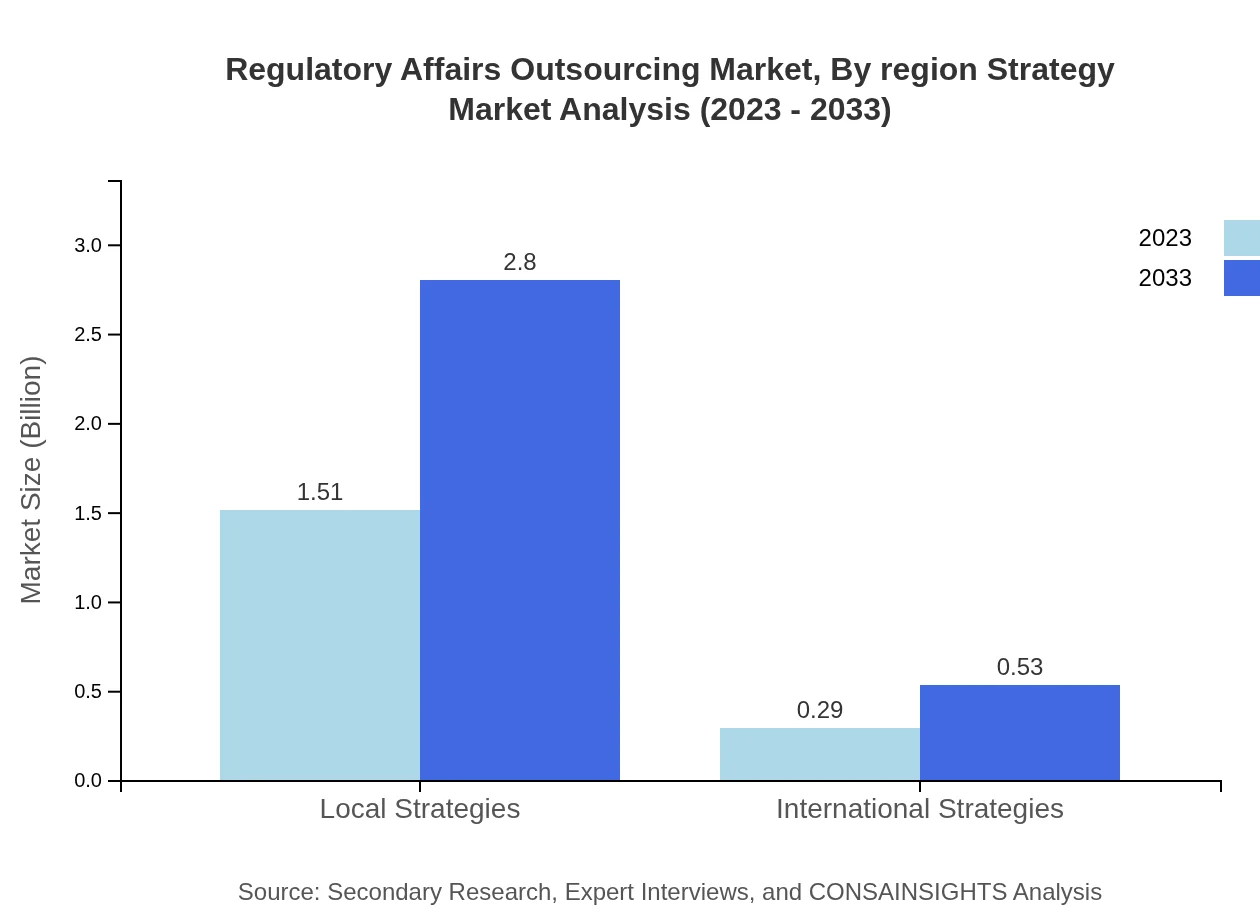
Regulatory Affairs Outsourcing Market Analysis By Region Strategy
Strategies in the market are categorized into Local and International. Local strategies attract 83.98% of the market share, while International stand at 16.02%, showing a preference for tailored strategies that address specific regulatory needs.
Regulatory Affairs Outsourcing Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Regulatory Affairs Outsourcing Industry
Covance:
Covance is a global leader in drug development and provides vital regulatory affairs outsourcing services, aiding clients in navigating complex regulatory landscapes.PAREXEL:
PAREXEL is prominent in providing comprehensive regulatory consulting services, helping companies align their products with global regulatory standards.ICON plc:
ICON plc specializes in outsourcing clinical development and regulatory affairs, focusing on ensuring compliance with local and international regulations.Charles River Laboratories:
Charles River offers comprehensive preclinical and clinical laboratory services, including crucial regulatory consulting that enhances drug development timelines.Regulatory Solutions:
Regulatory Solutions provides strategic consulting in regulatory affairs, offering tailored services for drugs, devices, and biologics.We're grateful to work with incredible clients.









FAQs
What is the market size of Regulatory Affairs Outsourcing?
The Regulatory Affairs Outsourcing market is estimated to reach $1.8 billion by 2023, with a projected CAGR of 6.2% over the next decade, indicating substantial growth potential in the sector.
What are the key market players or companies in the Regulatory Affairs Outsourcing industry?
Key players in the Regulatory Affairs Outsourcing industry include major pharmaceutical companies, regulatory consultants, and specialized outsourcing firms providing services across various sectors such as biotechnology, medical devices, and food supplements.
What are the primary factors driving the growth in the Regulatory Affairs Outsourcing industry?
Growth in the Regulatory Affairs Outsourcing industry is driven by increasing regulatory complexities, demand for faster product approvals, cost efficiencies through outsourcing, and rising global health concerns leading to innovation in pharmaceuticals and biotechnology.
Which region is the fastest Growing in the Regulatory Affairs Outsourcing market?
The fastest-growing region in the Regulatory Affairs Outsourcing market is North America, projected to grow from $0.58 billion in 2023 to $1.08 billion by 2033, reflecting a strong demand for regulatory support and compliance services.
Does ConsaInsights provide customized market report data for the Regulatory Affairs Outsourcing industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs and insights in the Regulatory Affairs Outsourcing industry, helping clients make informed strategic decisions based on market dynamics.
What deliverables can I expect from this Regulatory Affairs Outsourcing market research project?
Expect comprehensive deliverables including detailed market analysis, player profiles, regional insights, growth forecasts, segment breakdowns, and tailored recommendations that align with your business objectives.
What are the market trends of Regulatory Affairs Outsourcing?
Key trends in the Regulatory Affairs Outsourcing market include increased adoption of digital technologies, growing preference for outsourcing among SMEs, integration of regulatory software solutions, and rising focus on compliance and quality assurance.