Renal Artery Stent Market Report
Published Date: 31 January 2026 | Report Code: renal-artery-stent
Renal Artery Stent Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Renal Artery Stent market, focusing on current trends, market size, projections for growth, and challenges within the industry from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
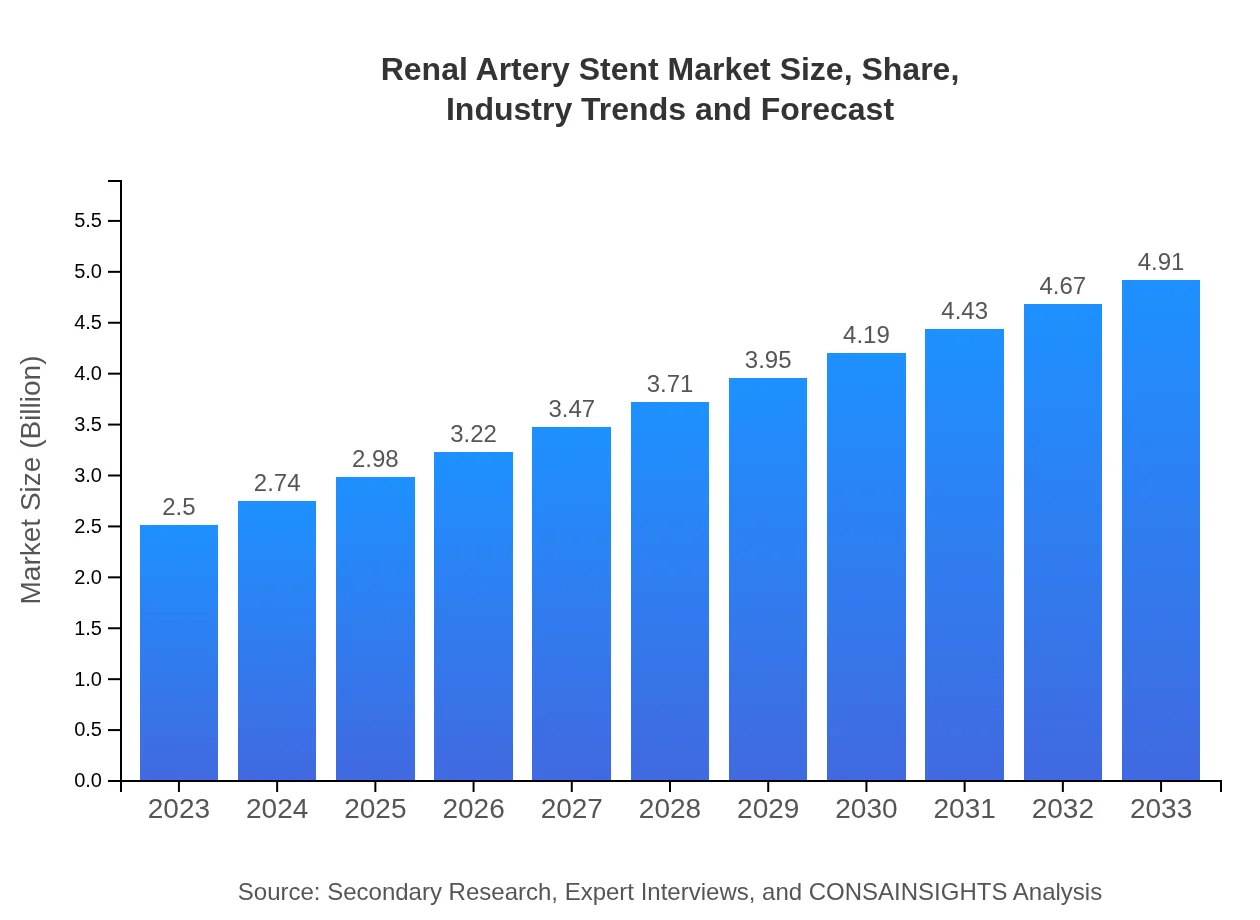
| 2023 Market Size | $2.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $4.91 Billion |
| Top Companies | Boston Scientific Corporation, Medtronic , Abbott Laboratories, B. Braun, Terumo Corporation |
| Last Modified Date | 31 January 2026 |
Renal Artery Stent Market Overview
Customize Renal Artery Stent Market Report market research report
- ✔ Get in-depth analysis of Renal Artery Stent market size, growth, and forecasts.
- ✔ Understand Renal Artery Stent's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Renal Artery Stent
What is the Market Size & CAGR of Renal Artery Stent market in 2033?
Renal Artery Stent Industry Analysis
Renal Artery Stent Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Renal Artery Stent Market Analysis Report by Region
Europe Renal Artery Stent Market Report:
The European Renal Artery Stent market was valued at $0.83 billion in 2023, with projections to reach $1.63 billion by 2033. Growing aging population, technological advancements, and support from healthcare policies are key contributors to this growth.Asia Pacific Renal Artery Stent Market Report:
In 2023, the Asia Pacific Renal Artery Stent market is valued at approximately $0.47 billion and is projected to grow to $0.92 billion by 2033. This increase is attributed to healthcare infrastructure improvements and rising awareness regarding renal diseases. Also, the expanding population base with risk factors encourages market growth.North America Renal Artery Stent Market Report:
North America is one of the leading regions in the Renal Artery Stent market, with a market value of $0.91 billion in 2023, expected to reach $1.78 billion by 2033. The high incidence of hypertension and robust healthcare infrastructure play a significant role in driving this growth.South America Renal Artery Stent Market Report:
The South American market for Renal Artery Stents is relatively modest, with a valuation of $0.10 billion in 2023, doubling to $0.20 billion by 2033. This growth will be driven by increasing healthcare expenditure and government initiatives to improve cardiovascular health.Middle East & Africa Renal Artery Stent Market Report:
In the Middle East and Africa, the market is projected to expand from $0.19 billion in 2023 to $0.38 billion by 2033. Rising awareness of cardiovascular health issues and improving access to healthcare services will spur market development.Tell us your focus area and get a customized research report.
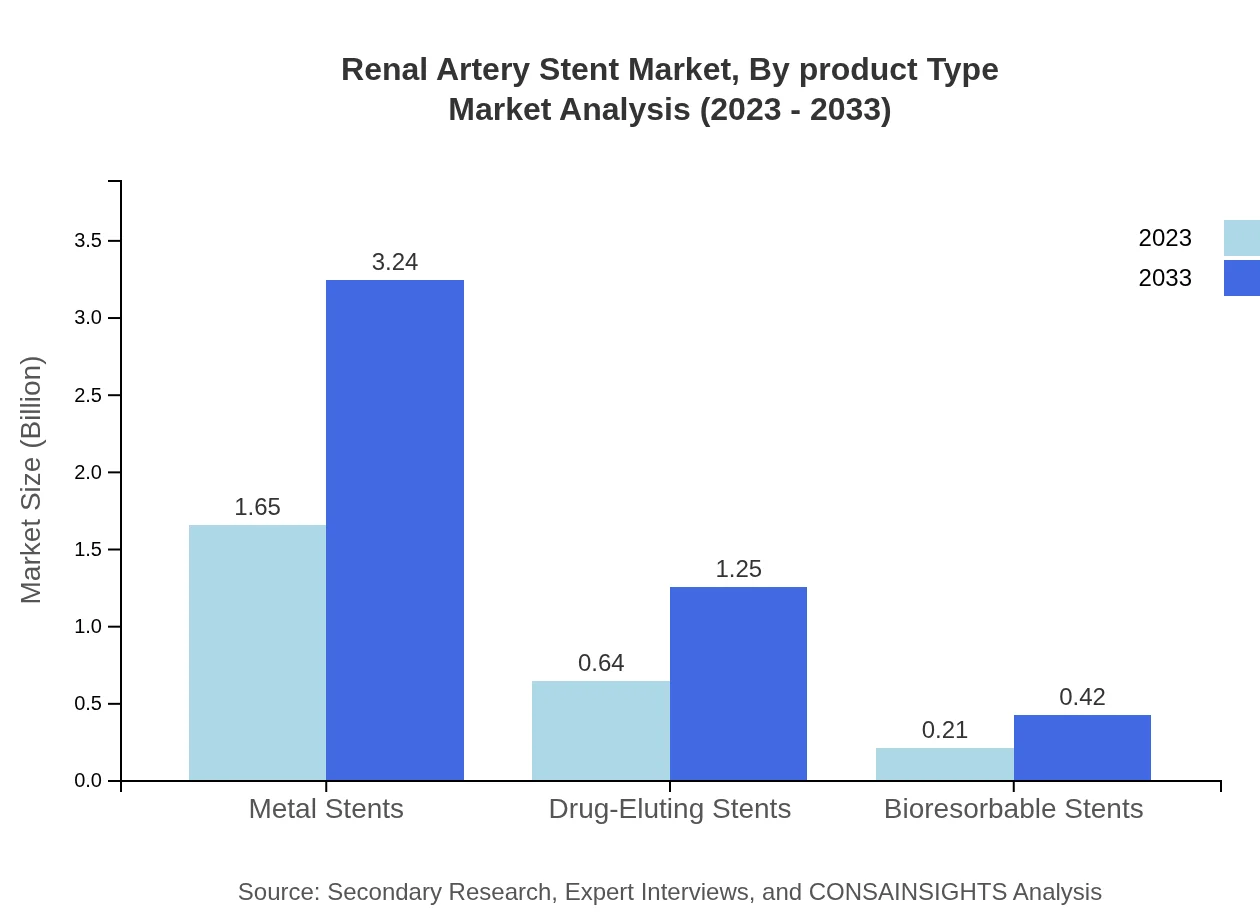
Renal Artery Stent Market Analysis By Product Type
The product types in the Renal Artery Stent market include metal stents, drug-eluting stents, and bioresorbable stents. Metal stents dominate this segment, accounting for 87.36% of the market in 2023 and projected to hold the same share in 2033, driven by their reliability and long-term performance. Drug-eluting stents are also gaining traction for their ability to reduce restenosis rates.
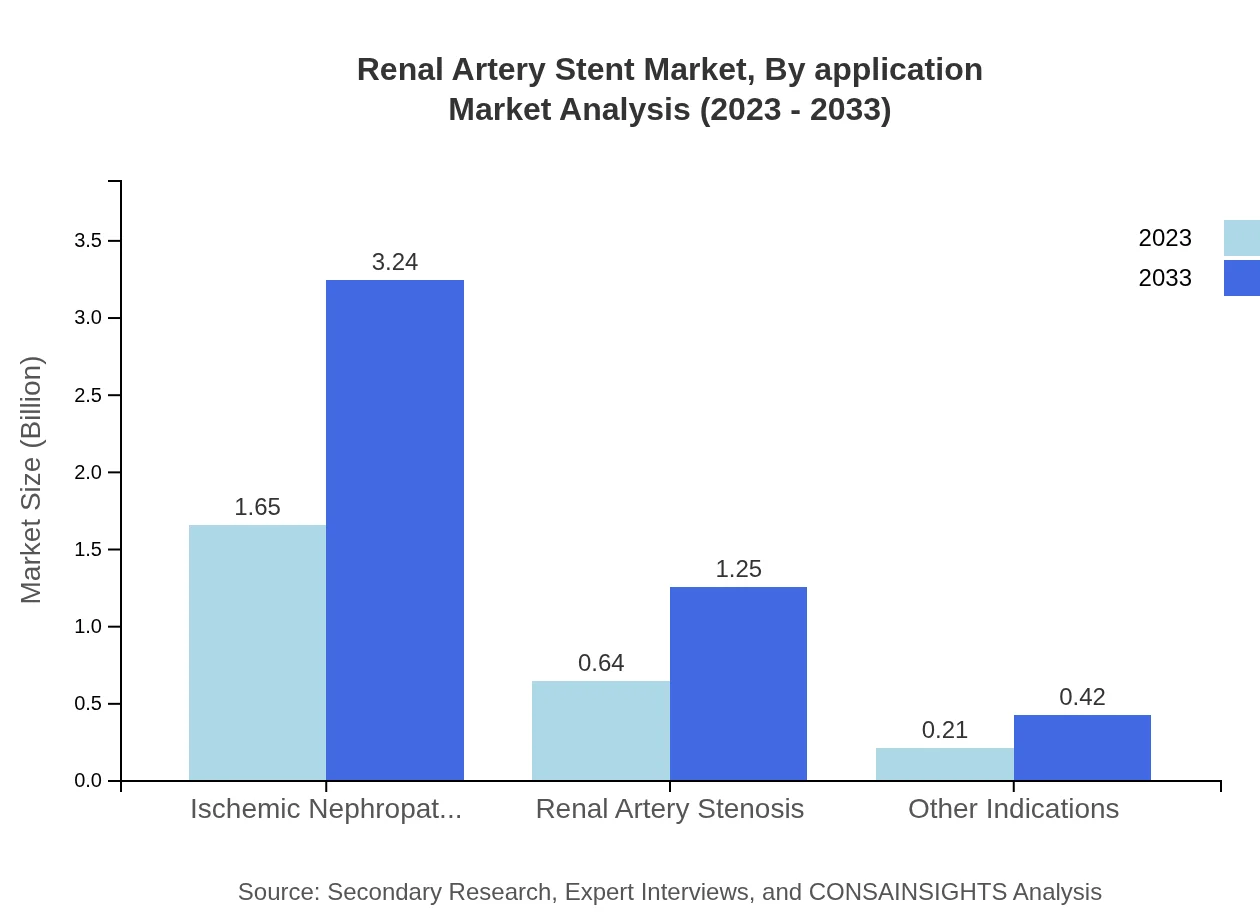
Renal Artery Stent Market Analysis By Application
The main applications encompass ischemic nephropathy, renal artery stenosis, and other indications. Ischemic nephropathy represents the largest share, holding 65.91% of the market in 2023 and maintaining this share through 2033. The growing patient population requiring effective therapies for renal artery stenosis is also significant, as it accounted for 25.49% of the market.
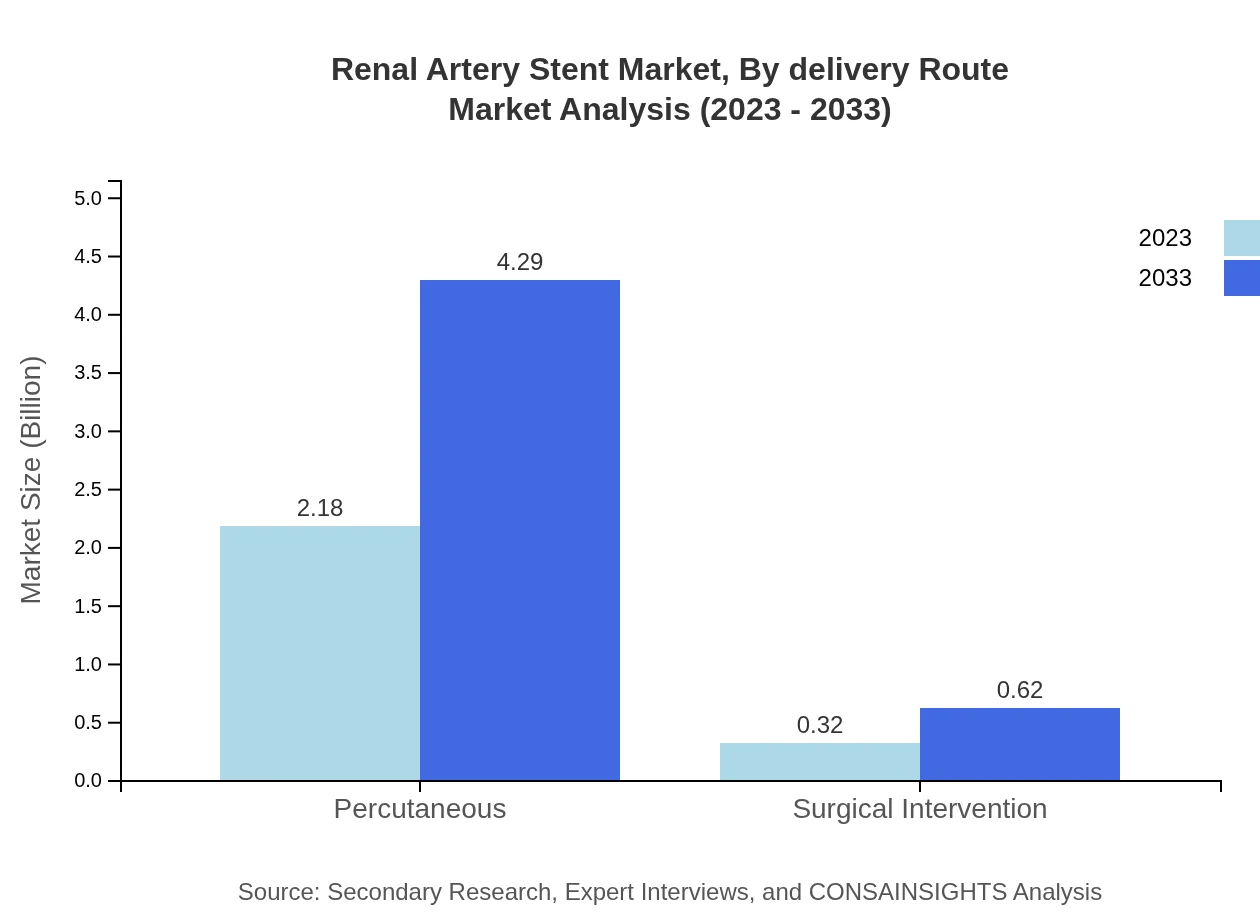
Renal Artery Stent Market Analysis By Delivery Route
In terms of delivery routes, percutaneous interventions hold a dominating market share of 87.36% in 2023 and will continue to do so into 2033. The non-invasive nature of these procedures ensures quick recovery times, making them preferable among healthcare providers and patients.
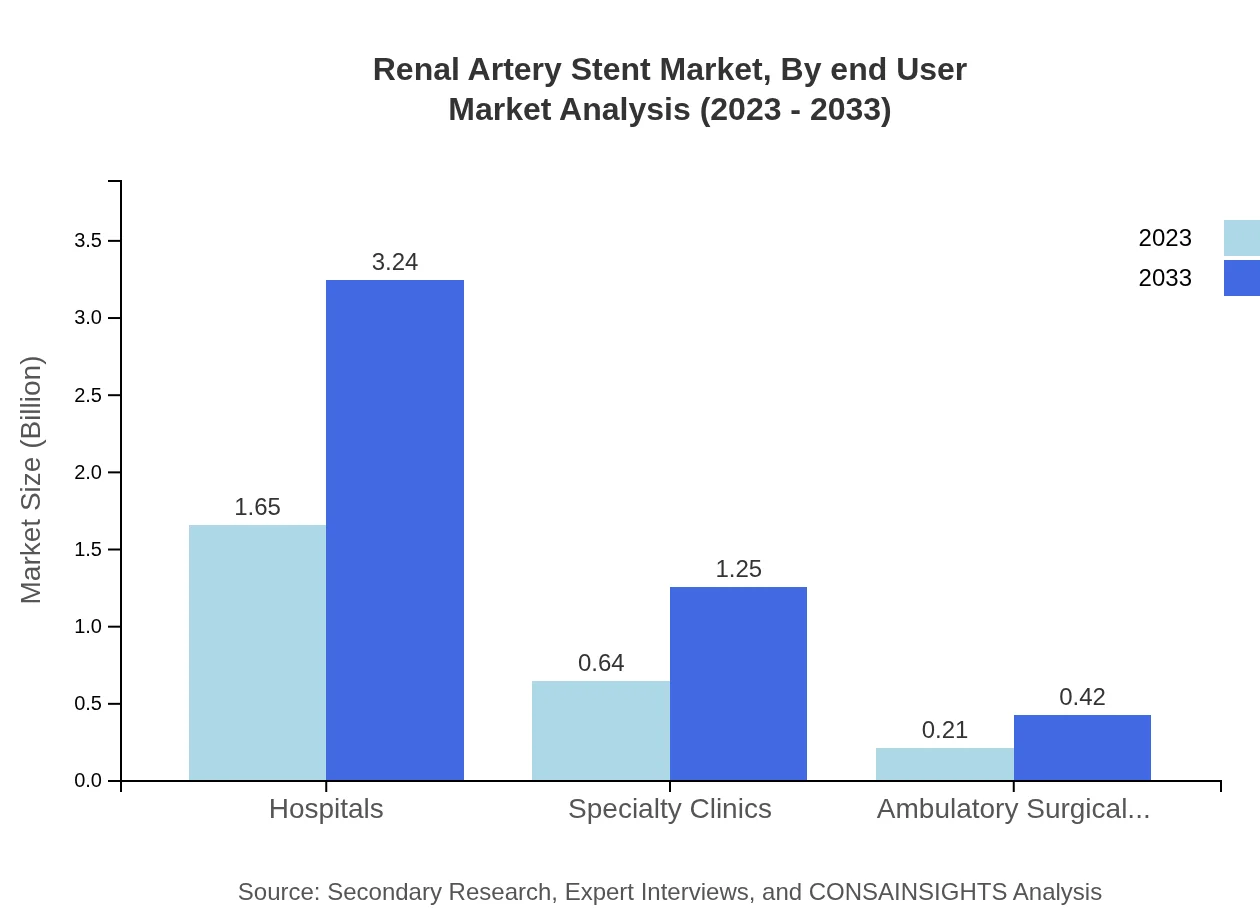
Renal Artery Stent Market Analysis By End User
The market can be segmented into hospitals, specialty clinics, and ambulatory surgical centers. Hospitals account for the majority share, comprising 65.91% in 2023. Specialty clinics and ambulatory surgical centers are also vital as they cater to specific procedures that require less overhead compared to traditional settings.
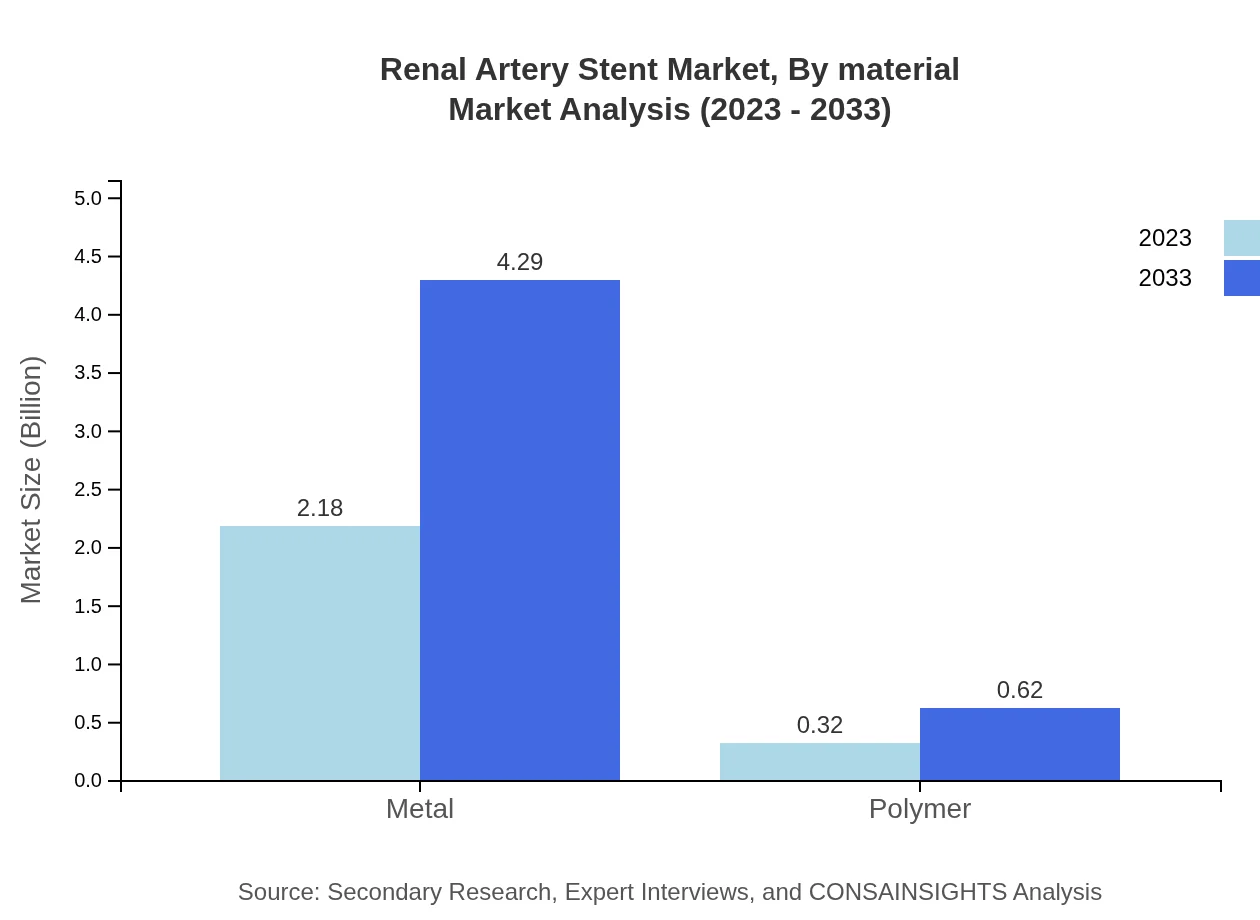
Renal Artery Stent Market Analysis By Material
The materials used in the production of renal artery stents include metals and polymers. Metal stents maintain a significant market share of 87.36% in 2023, attributed to their durability and effectiveness. Alternatively, polymer stents, while emerging, hold a smaller share of 12.64% but are expected to grow within the coming decade.
Renal Artery Stent Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Renal Artery Stent Industry
Boston Scientific Corporation:
A leading global developer of medical devices, Boston Scientific is at the forefront of innovation in renal artery stents, focusing on user-friendly designs and advanced materials.Medtronic :
Known for its diverse portfolio, Medtronic provides advanced stenting solutions and is heavily involved in ongoing research to enhance treatment efficacy for renal diseases.Abbott Laboratories:
Abbott specializes in drug-eluting stents and aggressively pursues investments in renal technologies to alleviate the burden of kidney-related health issues worldwide.B. Braun:
B. Braun has a presence in the renal industry with a focus on providing high-quality medical devices and has contributed significantly to the growth of stent design innovation.Terumo Corporation:
Terumo is a pioneer in healthcare innovation and has introduced several critically acclaimed devices, including advanced renal artery stents.We're grateful to work with incredible clients.









FAQs
What is the market size of renal Artery Stent?
The renal artery stent market is valued at approximately $2.5 billion in 2023, with an expected compound annual growth rate (CAGR) of 6.8%, projecting significant growth in demand by 2033.
What are the key market players or companies in the renal Artery Stent industry?
Key players in the renal artery stent market include major medical device companies specializing in vascular interventions. They continuously innovate their product offerings to enhance performance and patient outcomes.
What are the primary factors driving the growth in the renal Artery Stent industry?
The primary growth drivers for the renal artery stent market include the rising prevalence of ischemic nephropathy, advancements in stent technology, and increasing awareness about renal artery diseases, driving demand for effective treatment options.
Which region is the fastest Growing in the renal Artery Stent?
The fastest-growing region in the renal artery stent market is Europe, projected to grow from $0.83 billion in 2023 to $1.63 billion by 2033, driven by advancements in healthcare infrastructure.
Does ConsaInsights provide customized market report data for the renal Artery Stent industry?
Yes, ConsaInsights offers customized market report data specific to the renal artery stent industry, tailored to client requirements, which include comprehensive analysis and insights.
What deliverables can I expect from this renal Artery Stent market research project?
Expect comprehensive deliverables such as detailed market analysis, competitive landscape insights, forecasts, and recommendations based on rigorous data analysis to inform strategic decisions.
What are the market trends of renal Artery Stent?
Key trends in the renal artery stent market include a shift towards drug-eluting stents, increased adoption of minimally invasive procedures, and a growth in partnerships among healthcare providers and manufacturers.