Renal Biomarkers Market Report
Published Date: 31 January 2026 | Report Code: renal-biomarkers
Renal Biomarkers Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the renal biomarkers market, covering current trends, segmentation, and forecasts from 2023 to 2033. Insights include market size, growth rates, regional analysis, and profiles of key players.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
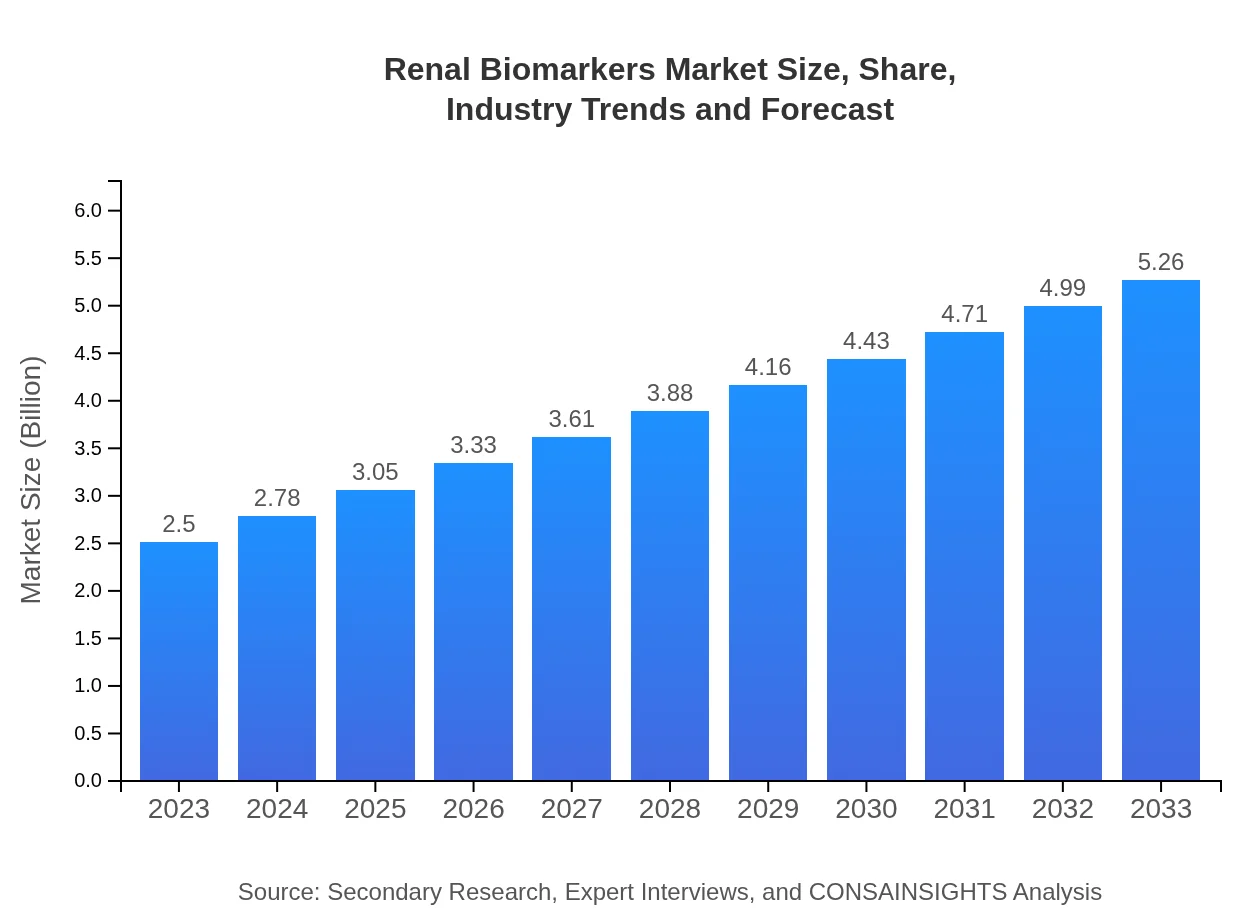
| 2023 Market Size | $2.50 Billion |
| CAGR (2023-2033) | 7.5% |
| 2033 Market Size | $5.26 Billion |
| Top Companies | Abbott Laboratories, Roche Diagnostics, Siemens Healthineers, Thermo Fisher Scientific |
| Last Modified Date | 31 January 2026 |
Renal Biomarkers Market Overview
Customize Renal Biomarkers Market Report market research report
- ✔ Get in-depth analysis of Renal Biomarkers market size, growth, and forecasts.
- ✔ Understand Renal Biomarkers's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Renal Biomarkers
What is the Market Size & CAGR of Renal Biomarkers market in 2033?
Renal Biomarkers Industry Analysis
Renal Biomarkers Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Renal Biomarkers Market Analysis Report by Region
Europe Renal Biomarkers Market Report:
Europe is another key market, starting at $0.91 billion in 2023 and projected to grow to $1.92 billion by 2033. Growth is fueled by increased healthcare expenditures, government initiatives targeting chronic diseases, and advancements in diagnostics. The increasing awareness of the importance of early diagnosis is expected to enhance market resilience.Asia Pacific Renal Biomarkers Market Report:
The Asia-Pacific region shows significant potential, with a market size of approximately $0.47 billion in 2023, projected to reach $1.00 billion by 2033. Growth drivers include increasing healthcare infrastructure, rising prevalence of renal diseases, and government initiatives towards health awareness. Additionally, a growing aging population is expected to increase the demand for renal health services.North America Renal Biomarkers Market Report:
North America holds a substantial market share, worth approximately $0.82 billion in 2023 and expected to rise to $1.73 billion by 2033. High consumer awareness, advanced healthcare facilities, and early adoption of innovative technologies significantly drive this growth. Moreover, robust research and development investments lead to a steady influx of new biomarkers in the market.South America Renal Biomarkers Market Report:
The South American market's growth is sluggish, with a size of -$0.02 billion in 2023, which is estimated to marginally decline to -$0.03 billion by 2033. The complications arise from less healthcare expenditure and availability of advanced diagnostic tools. However, untapped markets in countries like Brazil and Argentina present potential for development.Middle East & Africa Renal Biomarkers Market Report:
The Middle East and Africa market is valued at approximately $0.31 billion in 2023, and it is expected to increase to $0.65 billion by 2033. Growth in the region is mainly concentrated in the Gulf Cooperation Council (GCC) countries due to investments in healthcare infrastructure, making them a key area for future opportunities in renal biomarkers.Tell us your focus area and get a customized research report.
Renal Biomarkers Market Analysis By Type
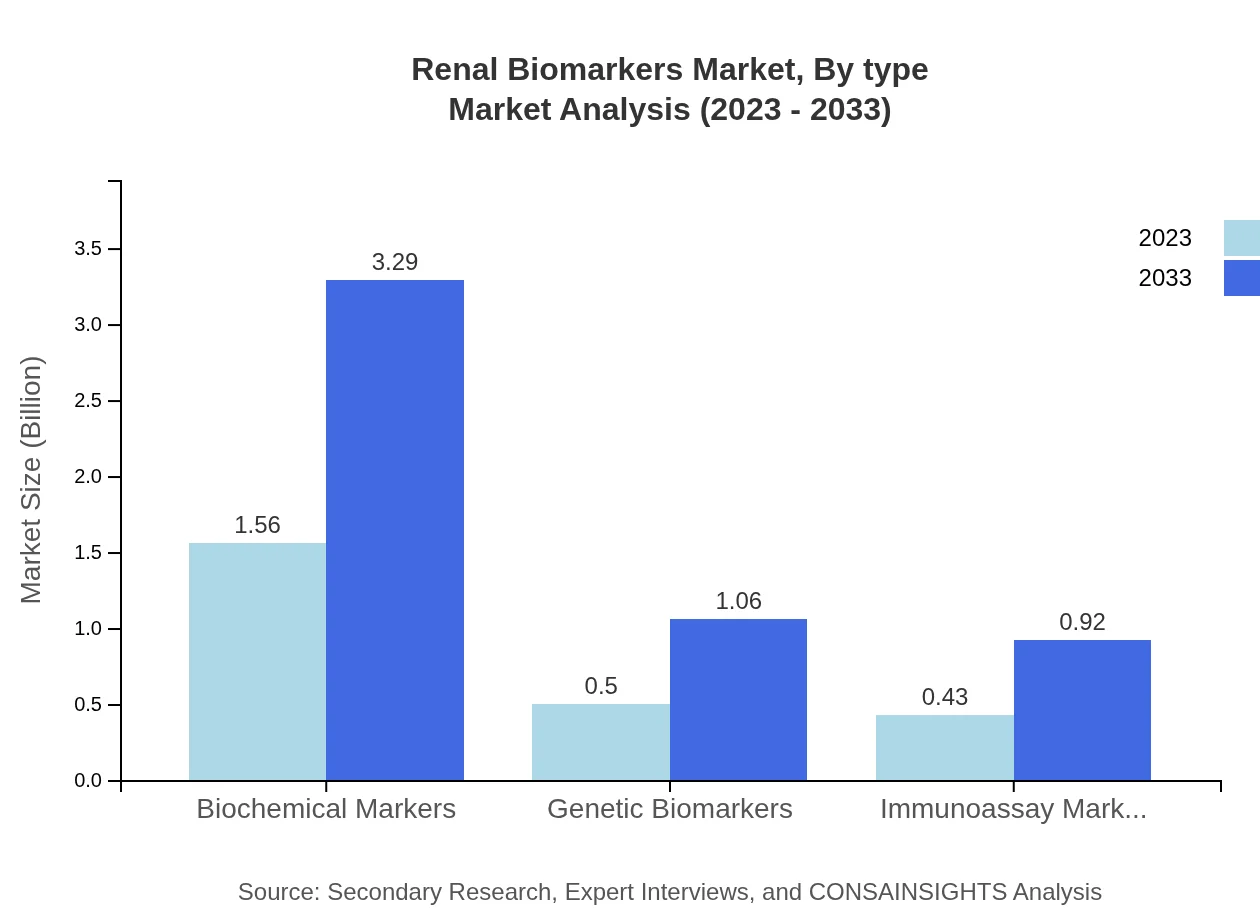
The segment includes diverse offerings like biochemical markers, genetic biomarkers, and immunoassay markers. Biochemical markers currently dominate the market with a size of $1.56 billion in 2023 and are expected to grow to $3.29 billion by 2033. Genetic biomarkers exhibit growth with the market projected to increase from $0.50 billion to $1.06 billion during the same period. Immunoassay markers also hold a significant market worth $0.43 billion in 2023, likely to reach $0.92 billion by 2033.
Renal Biomarkers Market Analysis By Application
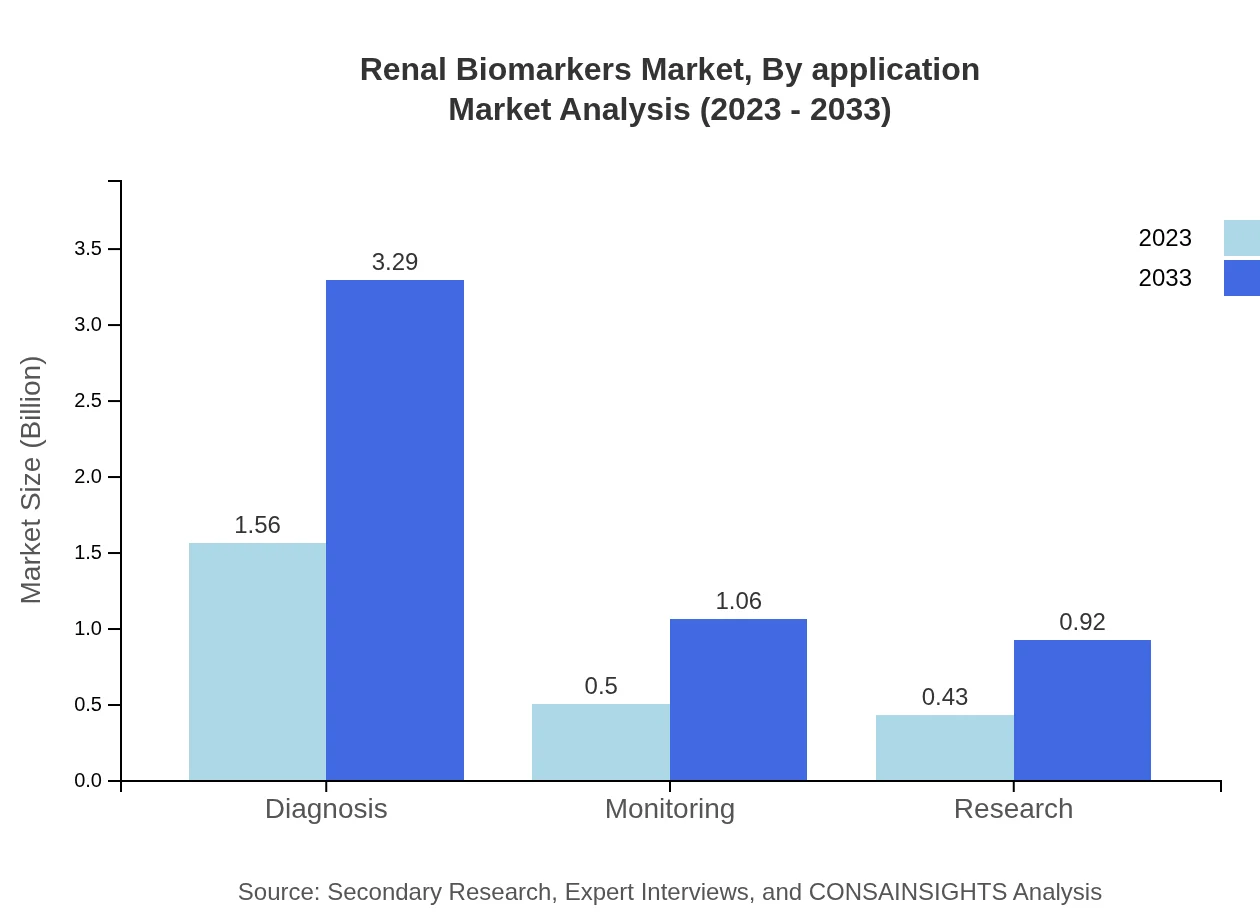
Applications are categorized primarily into diagnosis and monitoring. The diagnosis segment leads the market share at 62.42%, amounting to $1.56 billion in 2023, with a forecast to triple to $3.29 billion by 2033. The monitoring segment currently stands at $0.50 billion, expecting to grow to $1.06 billion within the forecast period.
Renal Biomarkers Market Analysis By Region
Global Renal Biomarkers Market, By Region Market Analysis (2023 - 2033)
Regional segmentation is critical, with North America, Europe, and Asia-Pacific comprising the bulk of market growth. North America leads with innovation and infrastructural readiness, while Europe boasts robust regulatory frameworks supporting new entrants. Asia-Pacific is rapidly catching up, showcasing potential for substantial growth due to increasing healthcare initiatives.
Renal Biomarkers Market Analysis By End User
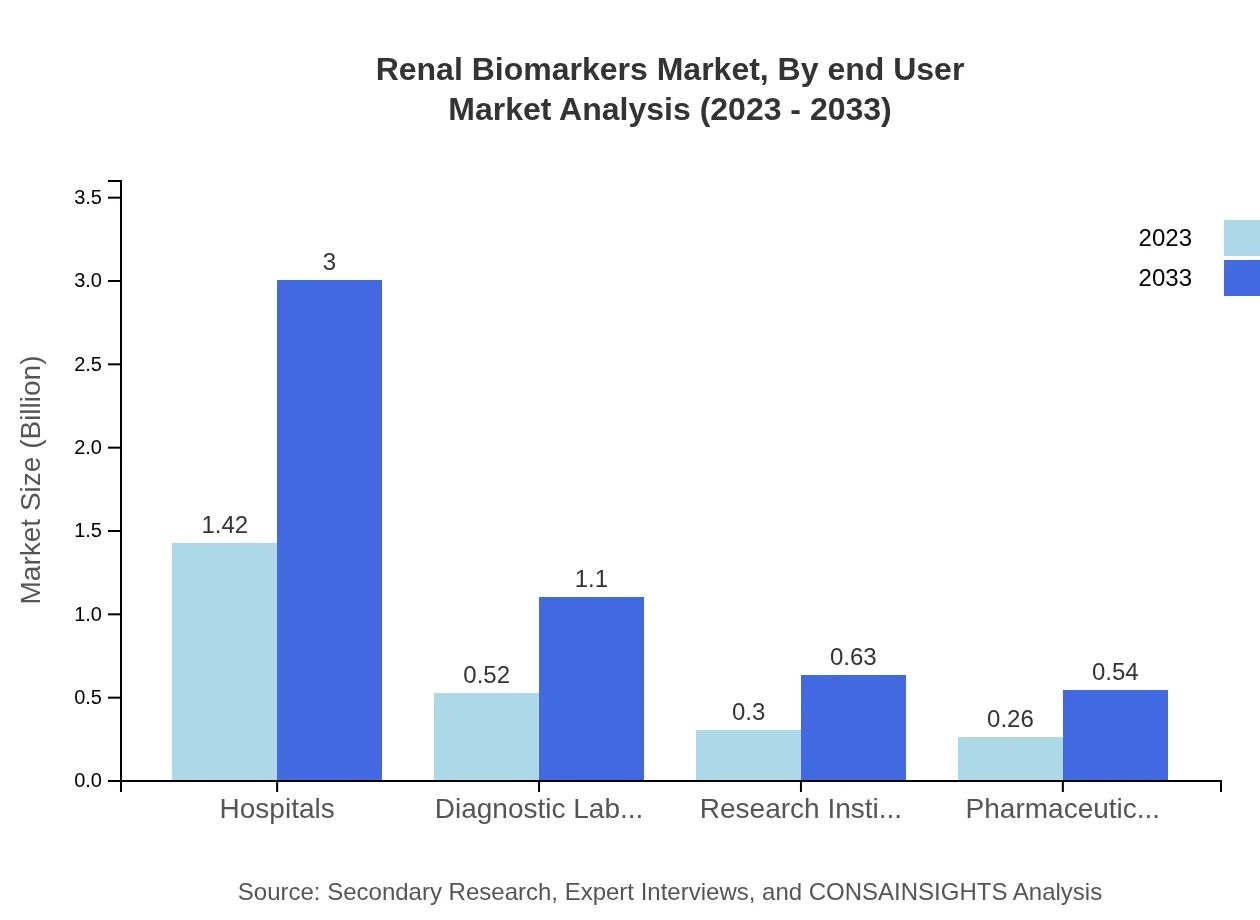
End-users encompass hospitals, diagnostic laboratories, research institutes, and pharmaceutical companies. Hospitals show promising growth as they hold the largest share of 56.91%, reflecting a size of $1.42 billion in 2023 and projected to reach $3.00 billion by 2033. Pharmaceutical companies, although smaller at $0.26 billion, are crucial in research and development initiatives.
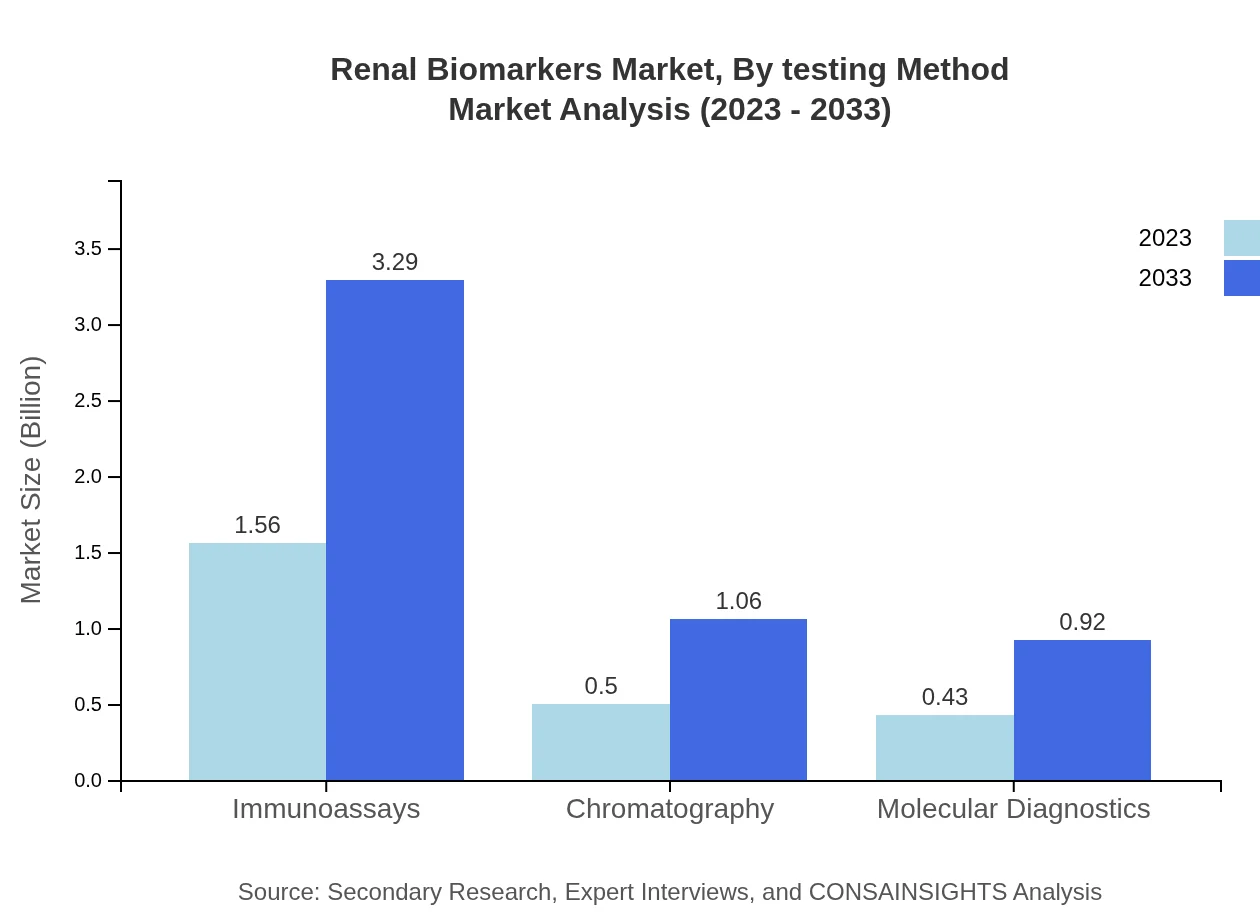
Renal Biomarkers Market Analysis By Testing Method
The testing methods include immunoassays, chromatography, and molecular diagnostics. Immunoassays dominate the segment, accounting for a market value of $1.56 billion in 2023, expected to rise to $3.29 billion by 2033. Chromatography and molecular diagnostics hold significant portions of the market, underscoring a shift towards technologically enhanced testing methods.
Renal Biomarkers Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Renal Biomarkers Industry
Abbott Laboratories:
A leading global healthcare company specializing in the development and manufacturing of medical devices and diagnostics, Abbott offers a wide range of renal biomarkers that assist in early disease detection and management.Roche Diagnostics:
Part of the Roche Group, this company focuses on innovative diagnostic solutions, including renal biomarkers that provide significant contributions to kidney disease diagnostics and patient care.Siemens Healthineers:
Known for its high-quality imaging and lab diagnostics solutions, Siemens Healthineers develops renal biomarkers that help in the precise monitoring and diagnosis of kidney-related conditions.Thermo Fisher Scientific:
A prominent player in the biotechnology sector, Thermo Fisher offers advanced diagnostic tools that are pivotal in the renal biomarkers market, aiming to improve patient outcomes through better diagnostics.We're grateful to work with incredible clients.









FAQs
What is the market size of renal Biomarkers?
The renal biomarkers market is valued at approximately $2.5 billion in 2023, with an impressive CAGR of 7.5% projected through 2033. This growth indicates increasing demand for advanced diagnostics and monitoring solutions in nephrology.
What are the key market players or companies in this renal Biomarkers industry?
Key market players in the renal biomarkers industry include leading companies such as Abbott Laboratories, F. Hoffmann-La Roche, Siemens Healthineers, and Thermo Fisher Scientific, which contribute significantly to innovation and market growth.
What are the primary factors driving the growth in the renal biomarkers industry?
The growth in the renal biomarkers industry is driven by rising incidences of chronic kidney diseases, advancements in biomarker technologies, increasing awareness regarding early diagnosis, and a growing patient pool requiring effective renal management.
Which region is the fastest Growing in the renal biomarkers?
The fastest-growing region in the renal biomarkers market is Europe, anticipated to grow from $0.91 billion in 2023 to $1.92 billion by 2033, reflecting a robust healthcare infrastructure and increased investments in diagnostic innovations.
Does ConsaInsights provide customized market report data for the renal Biomarkers industry?
Yes, Consainsights offers customized market report data tailored specifically to the renal biomarkers industry, allowing clients to obtain unique insights and actionable intelligence for informed decision-making.
What deliverables can I expect from this renal Biomarkers market research project?
Deliverables from the renal biomarkers market research project include comprehensive market analysis reports, detailed regional insights, segmentation data, trends analysis, and strategic recommendations to guide business growth.
What are the market trends of renal Biomarkers?
Current trends in the renal biomarkers market include increasing reliance on molecular diagnostics, a shift towards personalized medicine, the development of rapid testing kits, and the integration of AI technology in diagnostic processes.