Renal Denervation Devices Market Report
Published Date: 31 January 2026 | Report Code: renal-denervation-devices
Renal Denervation Devices Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Renal Denervation Devices market from 2023 to 2033, including insights on market size, trends, segmentation, regional analysis, and key players, helping stakeholders make informed decisions.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
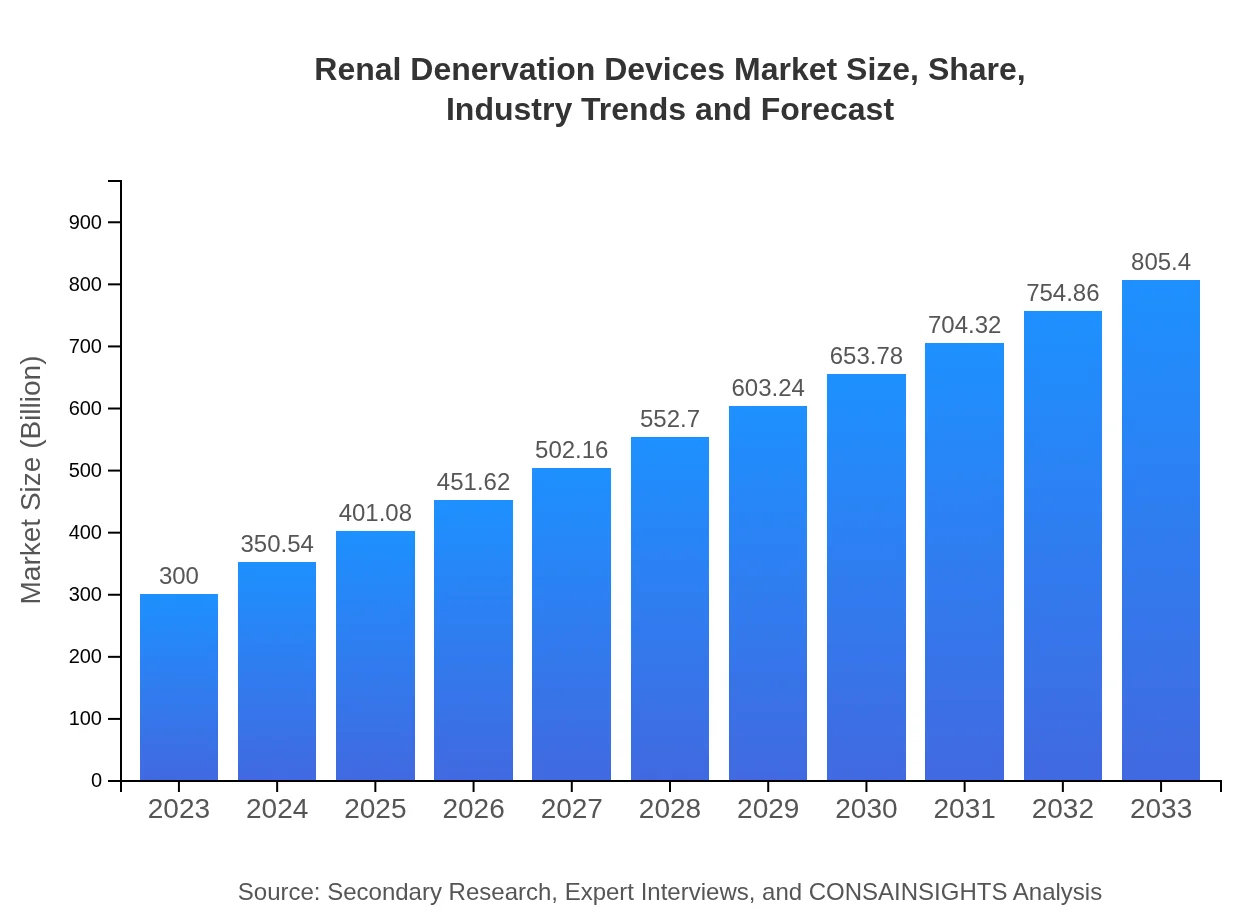
| 2023 Market Size | $300.00 Million |
| CAGR (2023-2033) | 10% |
| 2033 Market Size | $805.40 Million |
| Top Companies | Medtronic , Covidien (a Medtronic company), Abbott Laboratories, Recor Medical |
| Last Modified Date | 31 January 2026 |
Renal Denervation Devices Market Overview
Customize Renal Denervation Devices Market Report market research report
- ✔ Get in-depth analysis of Renal Denervation Devices market size, growth, and forecasts.
- ✔ Understand Renal Denervation Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Renal Denervation Devices
What is the Market Size & CAGR of Renal Denervation Devices market in 2023?
Renal Denervation Devices Industry Analysis
Renal Denervation Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Renal Denervation Devices Market Analysis Report by Region
Europe Renal Denervation Devices Market Report:
The European market is also witnessing growth, starting at $104.61 million in 2023 and expected to grow to $280.84 million by 2033. Strong healthcare frameworks and an aging population are primary drivers, alongside the increasing adoption of renal denervation procedures.Asia Pacific Renal Denervation Devices Market Report:
The Asia Pacific region is expected to grow significantly, with the market valued at $55.35 million in 2023 and projected to reach approximately $148.60 million by 2033. Increased awareness of hypertension management and investments in healthcare infrastructure are driving growth in countries like China and India.North America Renal Denervation Devices Market Report:
North America holds a dominant position, with a market size of $97.44 million in 2023, projected to reach $261.59 million by 2033. Innovations and a strong regulatory framework propel growth, coupled with a high prevalence of hypertension and chronic illnesses.South America Renal Denervation Devices Market Report:
In South America, the market size is estimated at $25.56 million in 2023, growing to around $68.62 million by 2033. The region faces challenges related to healthcare accessibility, but growing patient awareness and healthcare spending support market expansion.Middle East & Africa Renal Denervation Devices Market Report:
The Middle East and Africa region's market size is $17.04 million in 2023 and is poised to grow to $45.75 million by 2033. Investment in healthcare infrastructure and rising chronic disease prevalence are key factors driving this growth.Tell us your focus area and get a customized research report.
Renal Denervation Devices Market Analysis By Product
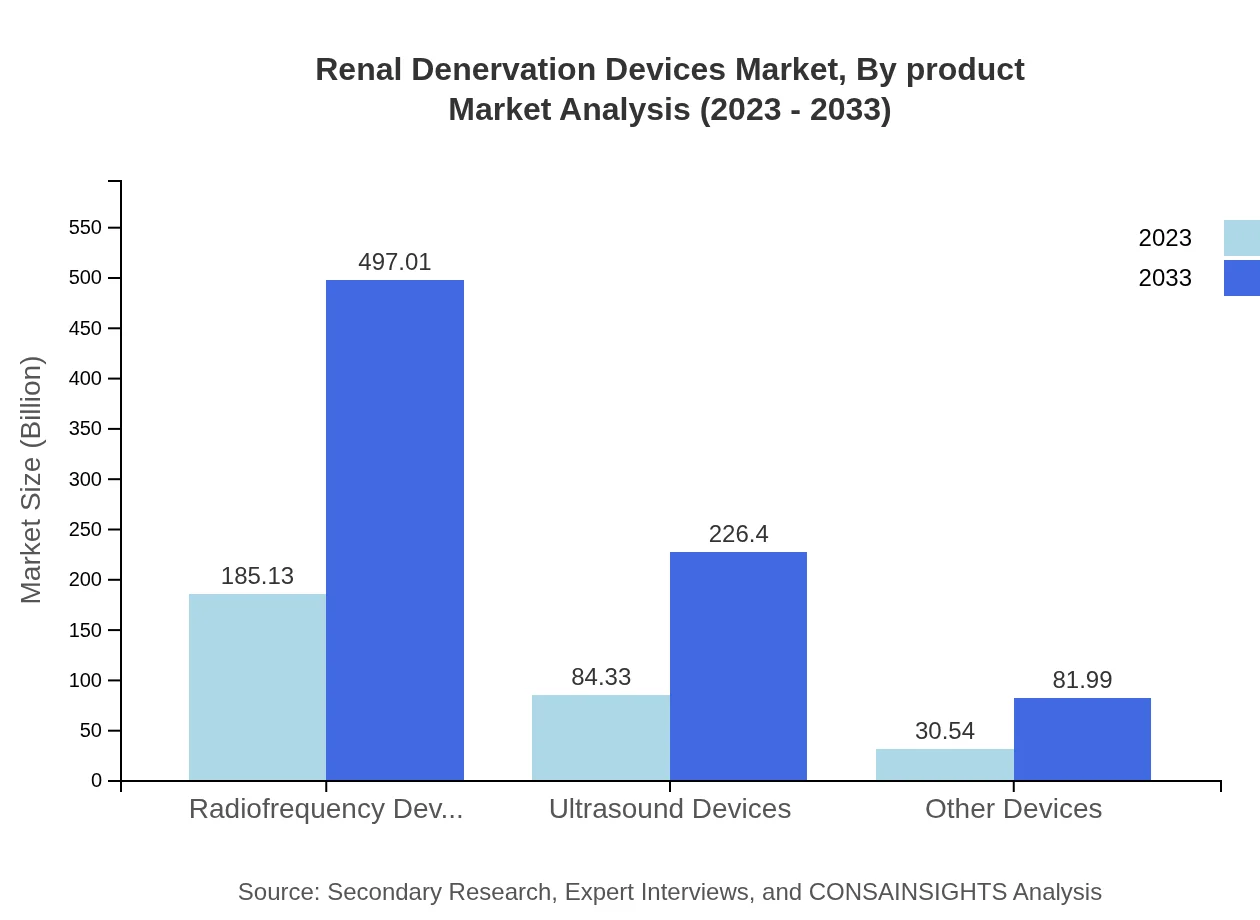
The market for Renal Denervation Devices by product is diverse, primarily segmented into radiofrequency, ultrasound, and other devices. In 2023, radiofrequency devices dominate with a market size of $185.13 million, expected to rise to $497.01 million by 2033. Ultrasound devices follow with a size of $84.33 million in 2023, projected to reach $226.40 million by 2033. Other devices account for $30.54 million and are anticipated to grow to $81.99 million in the same period, catering to various patient needs.
Renal Denervation Devices Market Analysis By Application
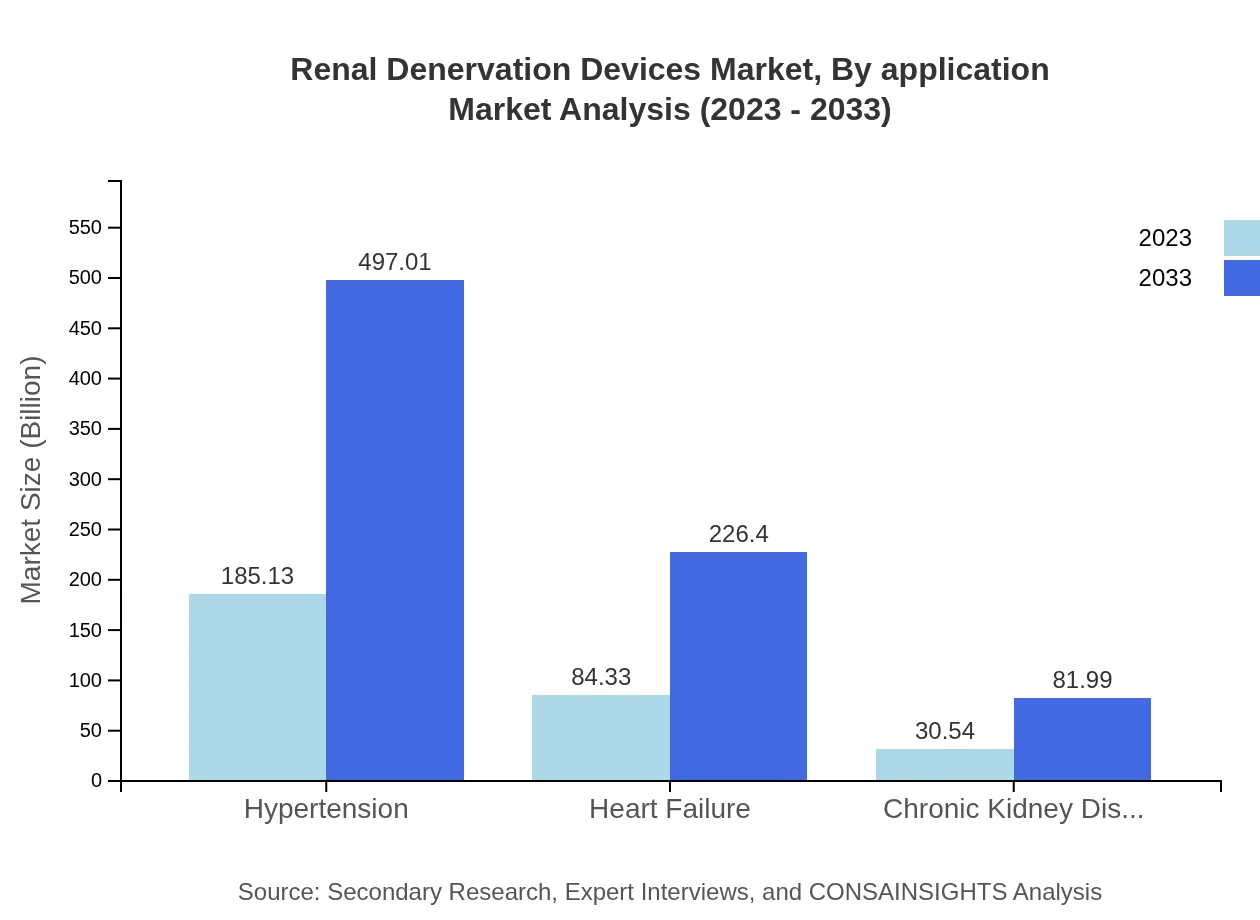
The application segment of Renal Denervation Devices consists mainly of hypertension, heart failure, and chronic kidney disease treatments. The hypertension application is the largest, valued at $185.13 million in 2023 and expected to grow to $497.01 million by 2033. Heart failure treatments begin at $84.33 million and will reach $226.40 million, while chronic kidney disease follow at $30.54 million with projection to $81.99 million, highlighting the market's response to increasing hypertension prevalence.
Renal Denervation Devices Market Analysis By End User
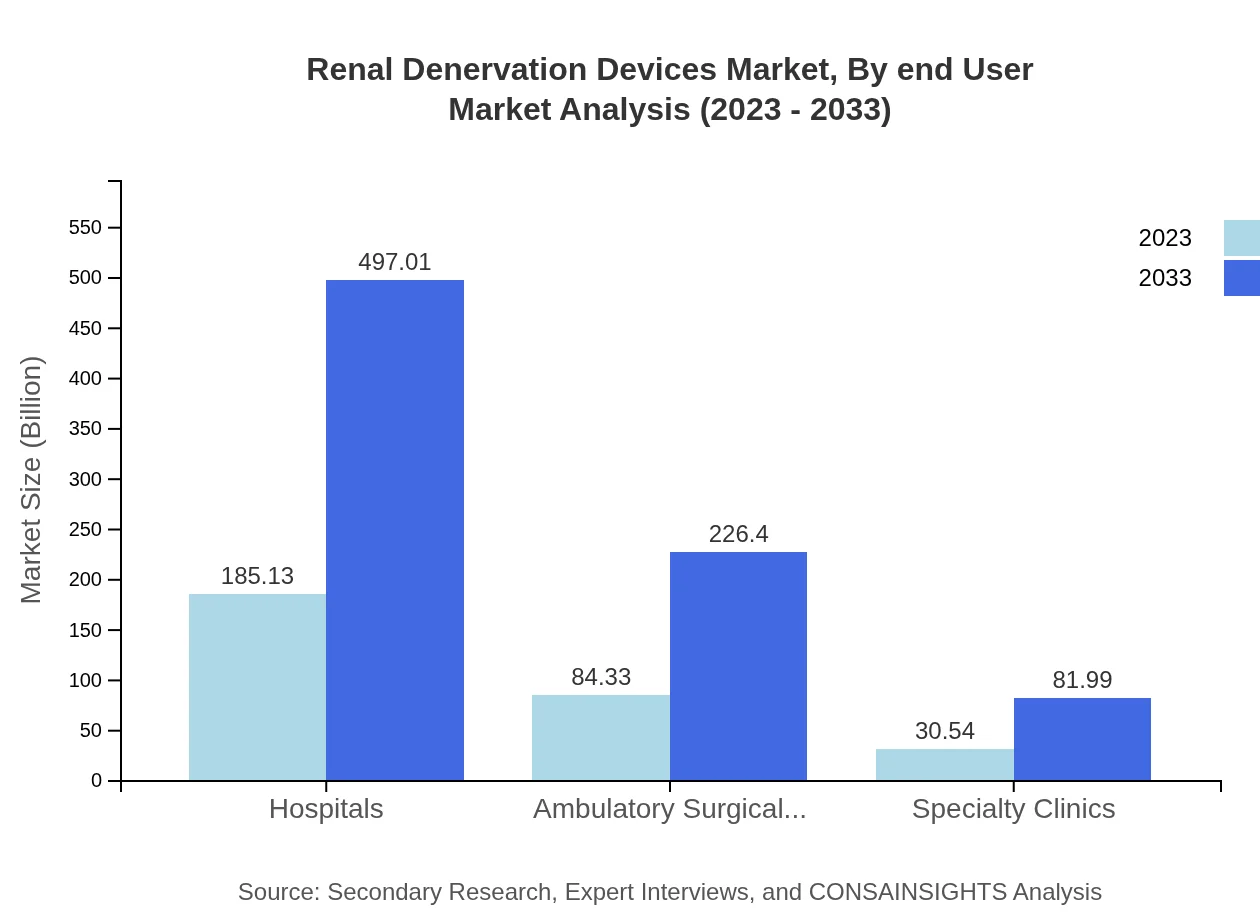
In terms of end-users, hospitals represent the dominant segment in the Renal Denervation Devices market, starting at $185.13 million in 2023 and expected to reach $497.01 million by 2033. Ambulatory surgical centers follow with $84.33 million and a projection of $226.40 million, while specialty clinics contribute a smaller share with $30.54 million, anticipating growth to $81.99 million as outpatient procedures increase.
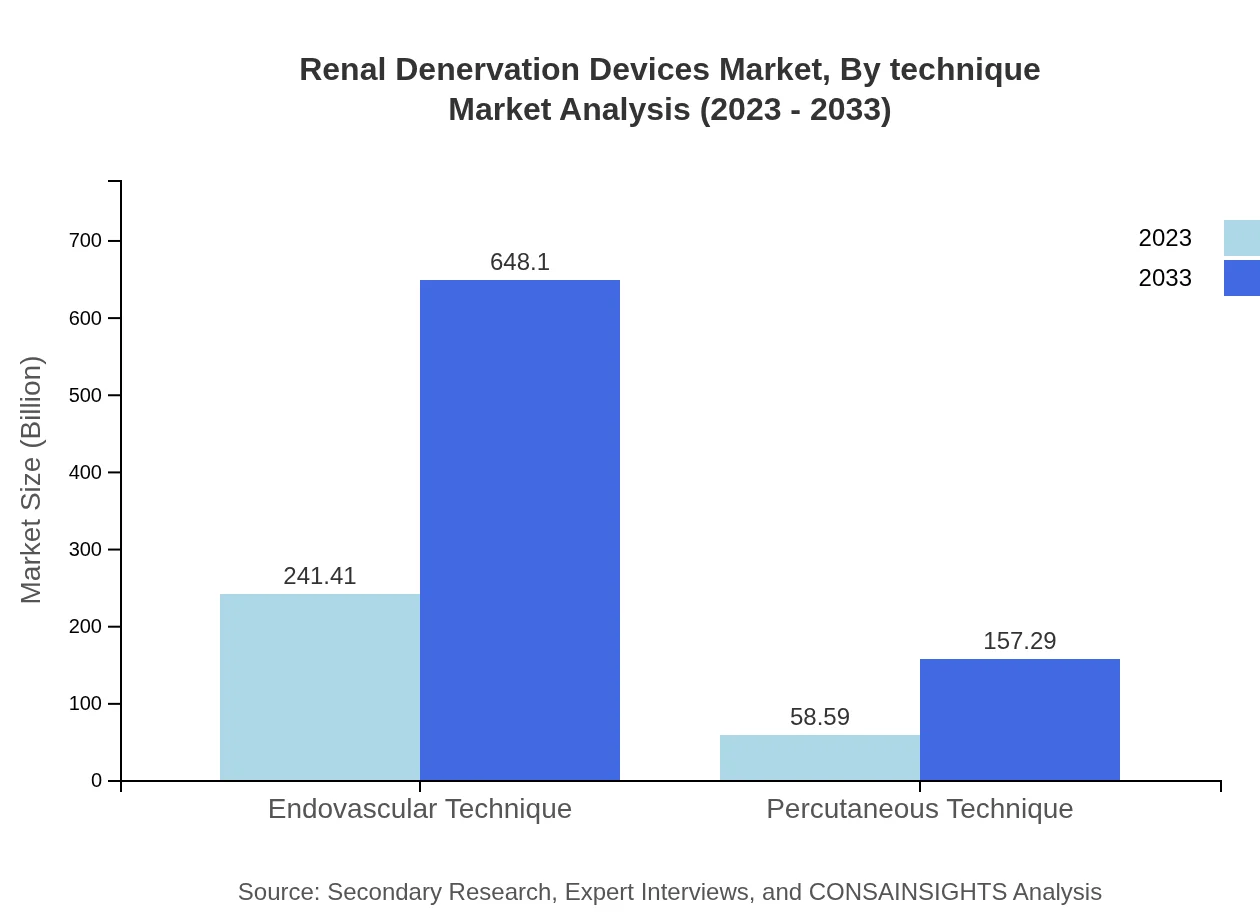
Renal Denervation Devices Market Analysis By Technique
The market segmentation by technique includes endovascular and percutaneous methods, both essential in renal denervation. Endovascular techniques dominate with a market size of $241.41 million expected to grow to $648.10 million by 2033, while percutaneous techniques begin at $58.59 million and are projected to reach $157.29 million. The rise of minimally invasive techniques has heightened the popularity of these procedures across healthcare settings.
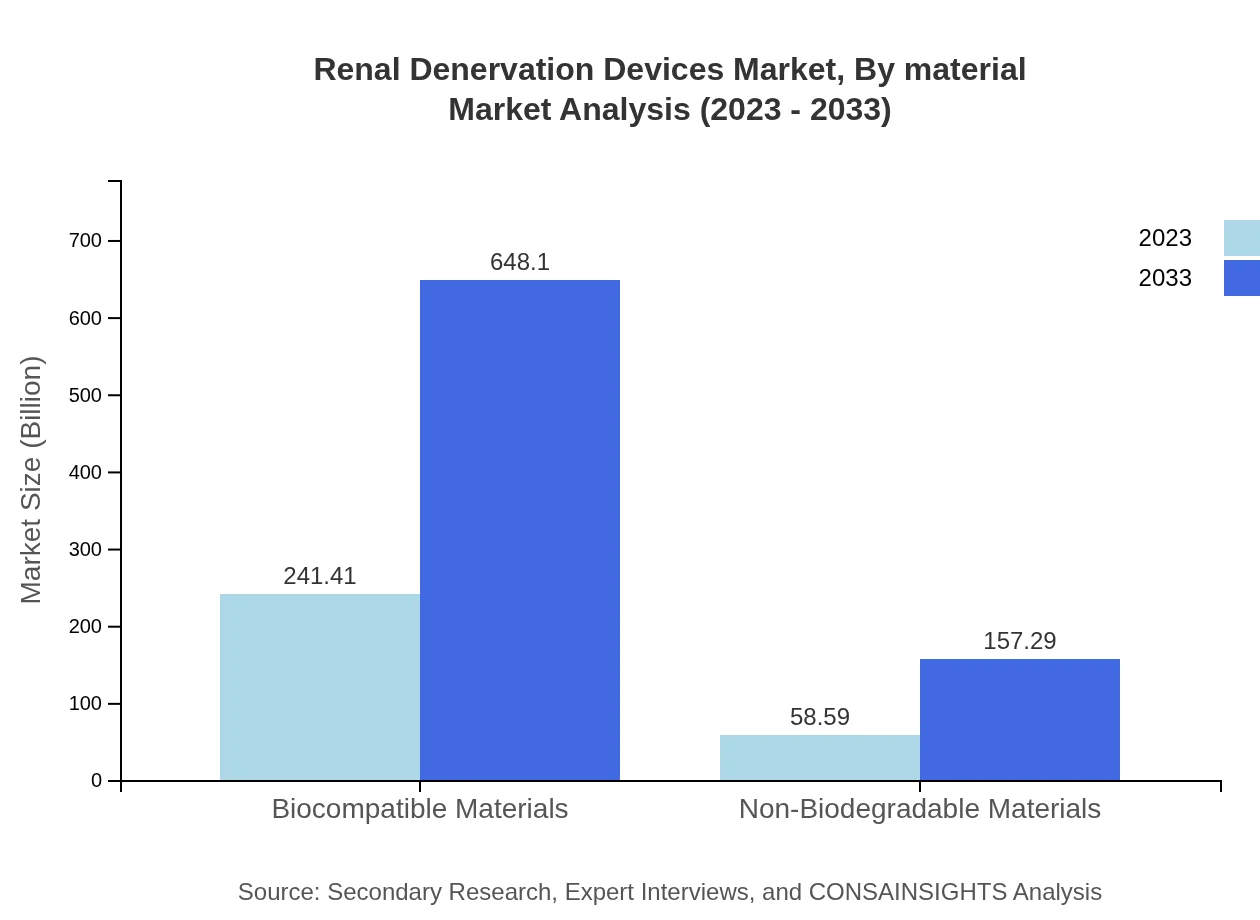
Renal Denervation Devices Market Analysis By Material
In the Renal Denervation Devices market, materials are categorized into biocompatible and non-biodegradable materials. Biocompatible materials have a significant lead, starting at $241.41 million in 2023 and projected to reach $648.10 million by 2033. Non-biodegradable materials contribute $58.59 million in 2023, expected to grow to $157.29 million. The increasing use of innovative materials tailored for improved patient outcomes supports demand.
Renal Denervation Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Renal Denervation Devices Industry
Medtronic :
A leading global healthcare solutions company specializing in medical devices, Medtronic is a pioneer in renal denervation technology, offering advanced products for hypertension treatment.Covidien (a Medtronic company):
Covidien, now part of Medtronic, is known for its innovative renal denervation systems and has significantly contributed to the advancement of minimally invasive techniques.Abbott Laboratories:
Abbott Laboratories focuses on healthcare innovation, developing robust renal denervation solutions aimed at addressing unmet health needs associated with kidney diseases.Recor Medical:
Specializing in renal denervation, Recor Medical designs cutting-edge systems with a focus on patient-centric solutions and enhancing treatment efficacy.We're grateful to work with incredible clients.









FAQs
What is the market size of renal Denervation Devices?
The renal denervation devices market was valued at approximately $300 million in 2023 and is projected to grow at a CAGR of 10%, reaching significant milestones leading up to 2033.
What are the key market players or companies in this renal Denervation Devices industry?
Key players in the renal denervation devices market include leading medical technology companies that specialize in hypertension treatments and renal therapies, contributing to innovative device development.
What are the primary factors driving the growth in the renal Denervation Devices industry?
Factors driving growth in this industry include rising hypertension prevalence, advancements in medical technology, increasing patient awareness, and favorable regulatory frameworks, encouraging innovations in treatment options.
Which region is the fastest Growing in the renal Denervation Devices?
Asia Pacific is the fastest-growing region in the renal denervation devices market, with market size projected to increase from $55.35 million in 2023 to $148.60 million by 2033.
Does ConsaInsights provide customized market report data for the renal Denervation Devices industry?
Yes, ConsaInsights offers customized market report data for the renal-denervation-devices industry, allowing stakeholders to tailor insights based on specific needs and regional dynamics.
What deliverables can I expect from this renal Denervation Devices market research project?
Expect deliverables such as comprehensive market analysis reports, segmented data by technology and application, competitive landscape insights, and forecasts for 2023 to 2033.
What are the market trends of renal Denervation Devices?
Current trends include increasing adoption of catheter-based therapies, rising investments in R&D for new devices, and a focus on minimally invasive procedures to enhance patient outcomes.