Respiratory Infectious Disease Diagnostics Market Report
Published Date: 31 January 2026 | Report Code: respiratory-infectious-disease-diagnostics
Respiratory Infectious Disease Diagnostics Market Size, Share, Industry Trends and Forecast to 2033
This report covers a comprehensive analysis of the Respiratory Infectious Disease Diagnostics market from 2023 to 2033, including insights on market size, growth rates, industry trends, and regional dynamics affecting the sector.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
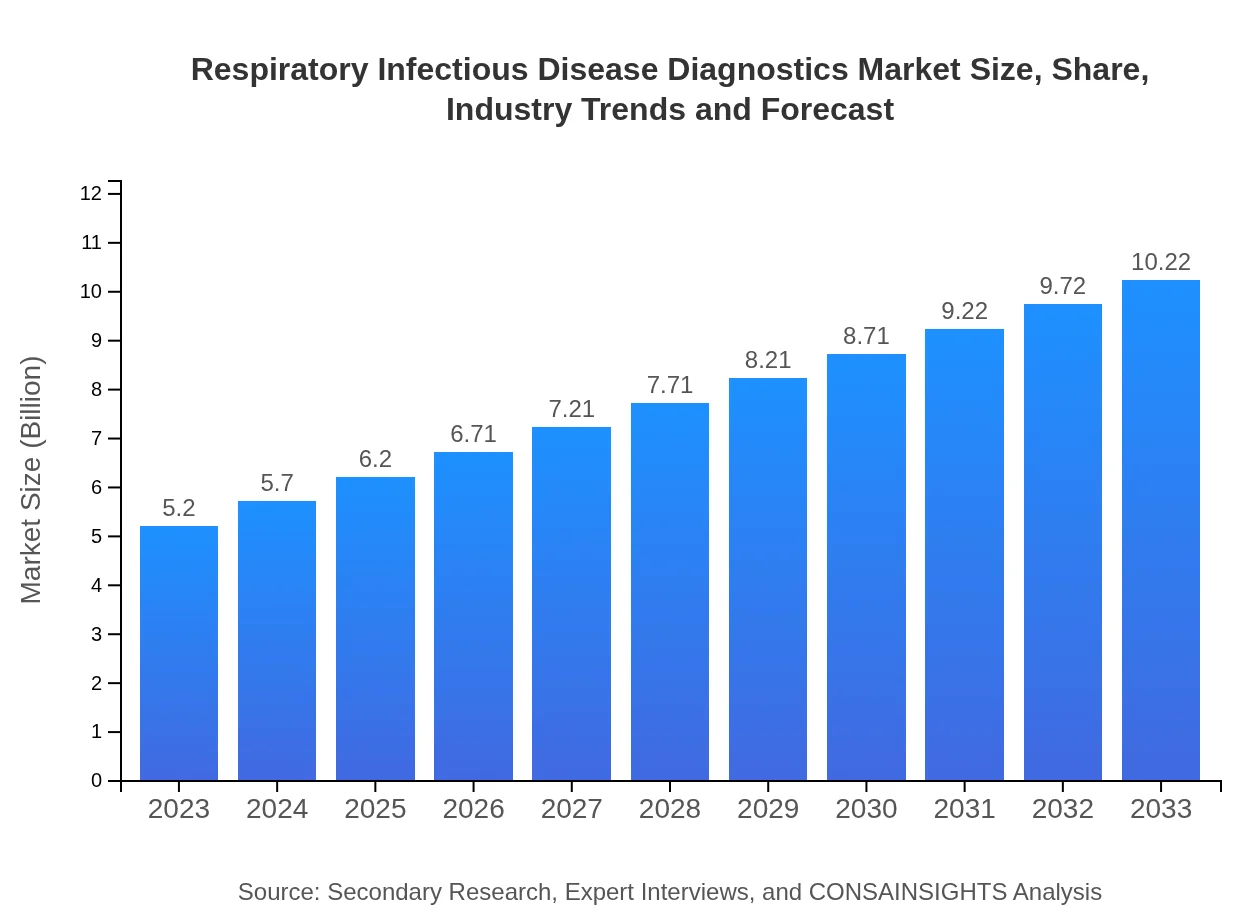
| 2023 Market Size | $5.20 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $10.22 Billion |
| Top Companies | Thermo Fisher Scientific, Roche Diagnostics, Siemens Healthineers, Abbott Laboratories, BD (Becton, Dickinson and Company) |
| Last Modified Date | 31 January 2026 |
Respiratory Infectious Disease Diagnostics Market Overview
Customize Respiratory Infectious Disease Diagnostics Market Report market research report
- ✔ Get in-depth analysis of Respiratory Infectious Disease Diagnostics market size, growth, and forecasts.
- ✔ Understand Respiratory Infectious Disease Diagnostics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Respiratory Infectious Disease Diagnostics
What is the Market Size & CAGR of Respiratory Infectious Disease Diagnostics market in 2023?
Respiratory Infectious Disease Diagnostics Industry Analysis
Respiratory Infectious Disease Diagnostics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Respiratory Infectious Disease Diagnostics Market Analysis Report by Region
Europe Respiratory Infectious Disease Diagnostics Market Report:
In Europe, the market is predicted to rise from $1.61 billion to $3.16 billion, reflecting ongoing investments in healthcare technology and robust support from regulatory bodies.Asia Pacific Respiratory Infectious Disease Diagnostics Market Report:
The Asia Pacific region's market is anticipated to expand from $0.89 billion in 2023 to $1.75 billion by 2033, reflecting a strong CAGR due to rising healthcare investments and the growing burden of respiratory diseases.North America Respiratory Infectious Disease Diagnostics Market Report:
North America is expected to grow from $2.02 billion in 2023 to $3.97 billion by 2033, supported by advanced healthcare systems and increasing awareness of respiratory health.South America Respiratory Infectious Disease Diagnostics Market Report:
South America exhibits potential growth but currently shows a decline in market size, projected to decrease from -$0.01 billion in 2023 to -$0.03 billion by 2033, primarily affected by infrastructural challenges.Middle East & Africa Respiratory Infectious Disease Diagnostics Market Report:
The Middle East and Africa market will likely grow from $0.69 billion in 2023 to $1.36 billion by 2033, spurred by improvements in healthcare infrastructure and the increasing incidence of respiratory diseases.Tell us your focus area and get a customized research report.
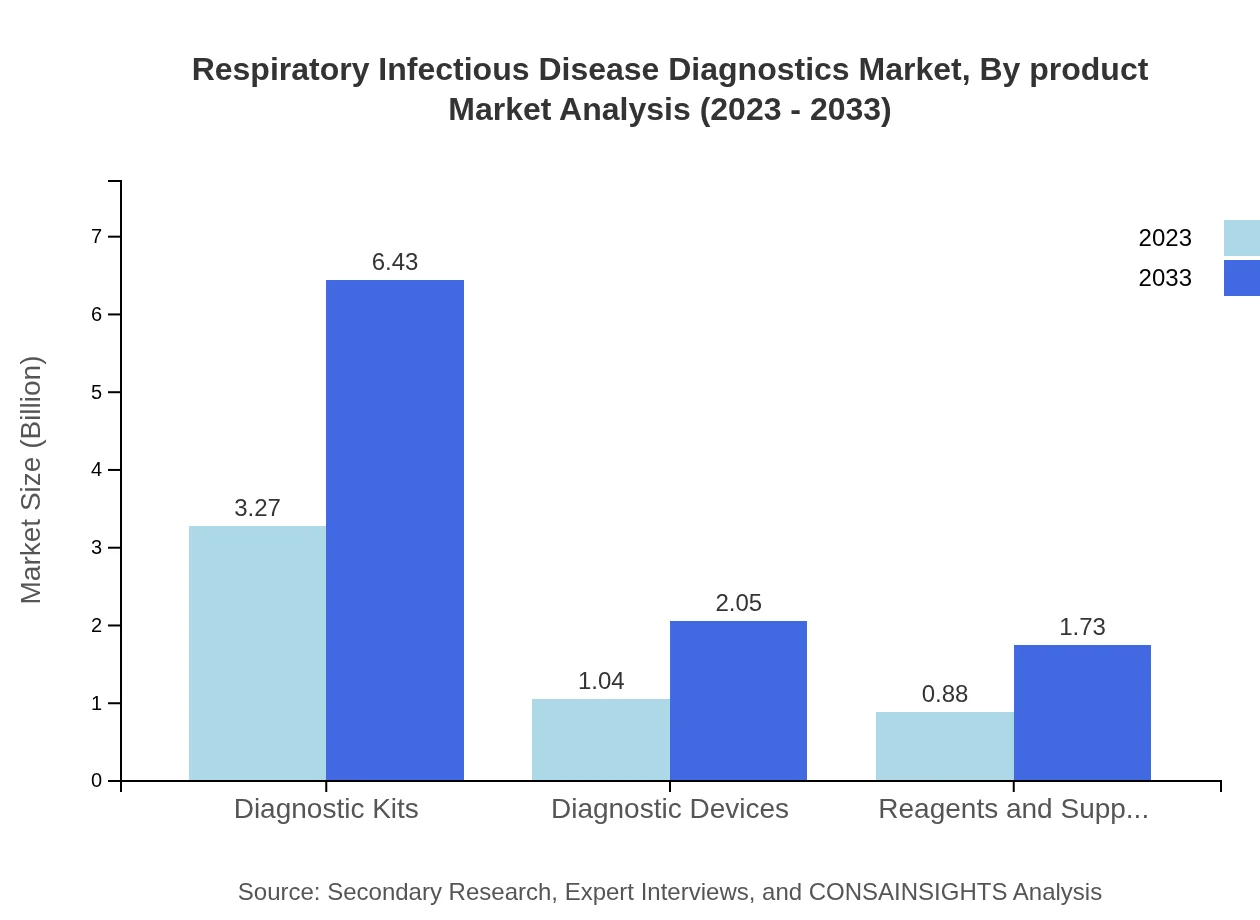
Respiratory Infectious Disease Diagnostics Market Analysis By Product
The product segment demonstrates significant diversity, with diagnostic kits leading the market. Projected values show diagnostic kits increasing from $3.27 billion in 2023 to $6.43 billion in 2033. Diagnostic devices and reagents hold substantial scopes as well, portraying strong growth trajectories.
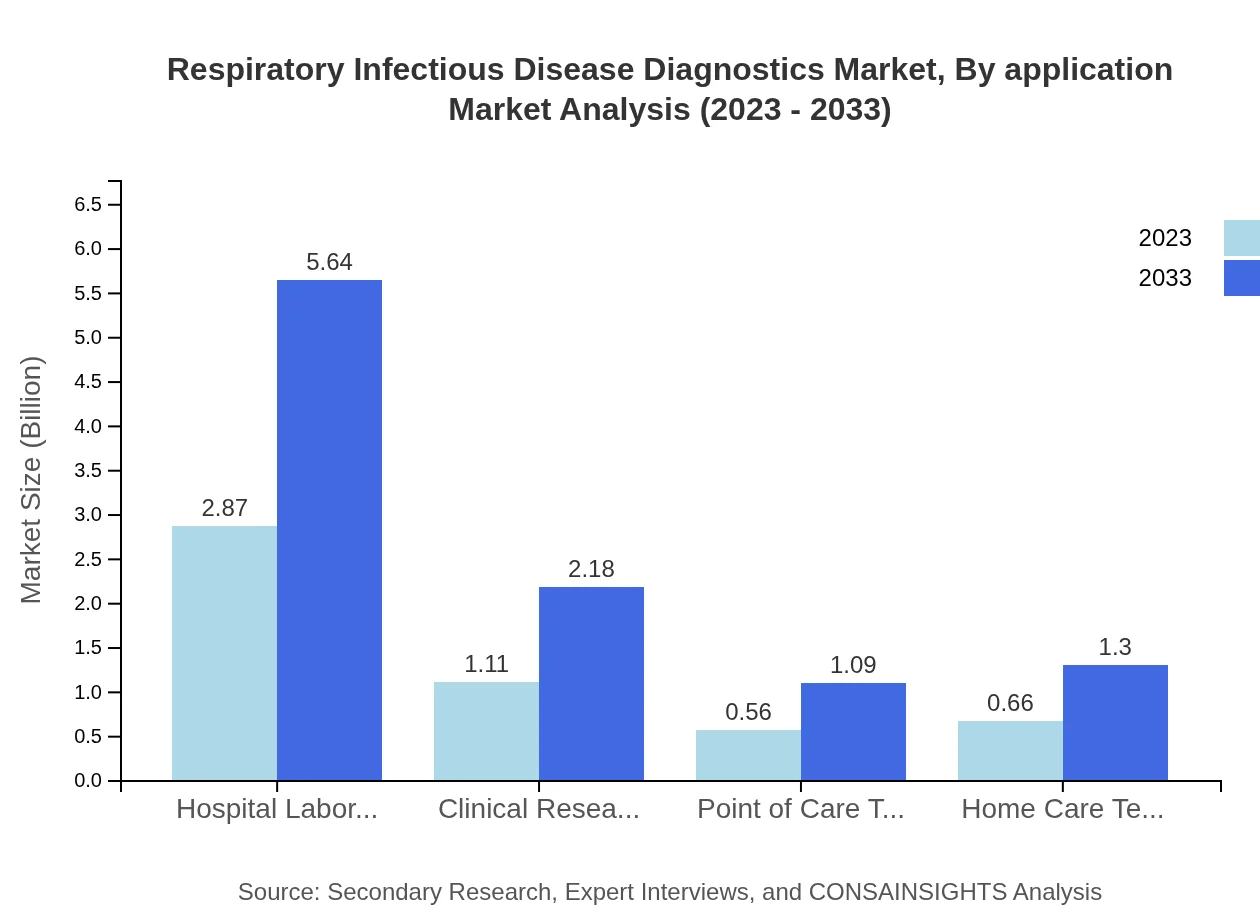
Respiratory Infectious Disease Diagnostics Market Analysis By Application
Applications primarily center on hospital laboratories, which are anticipated to show consistent growth from $2.87 billion to $5.64 billion. These applications underscore the vital role of accurate diagnostics in clinical outcomes.
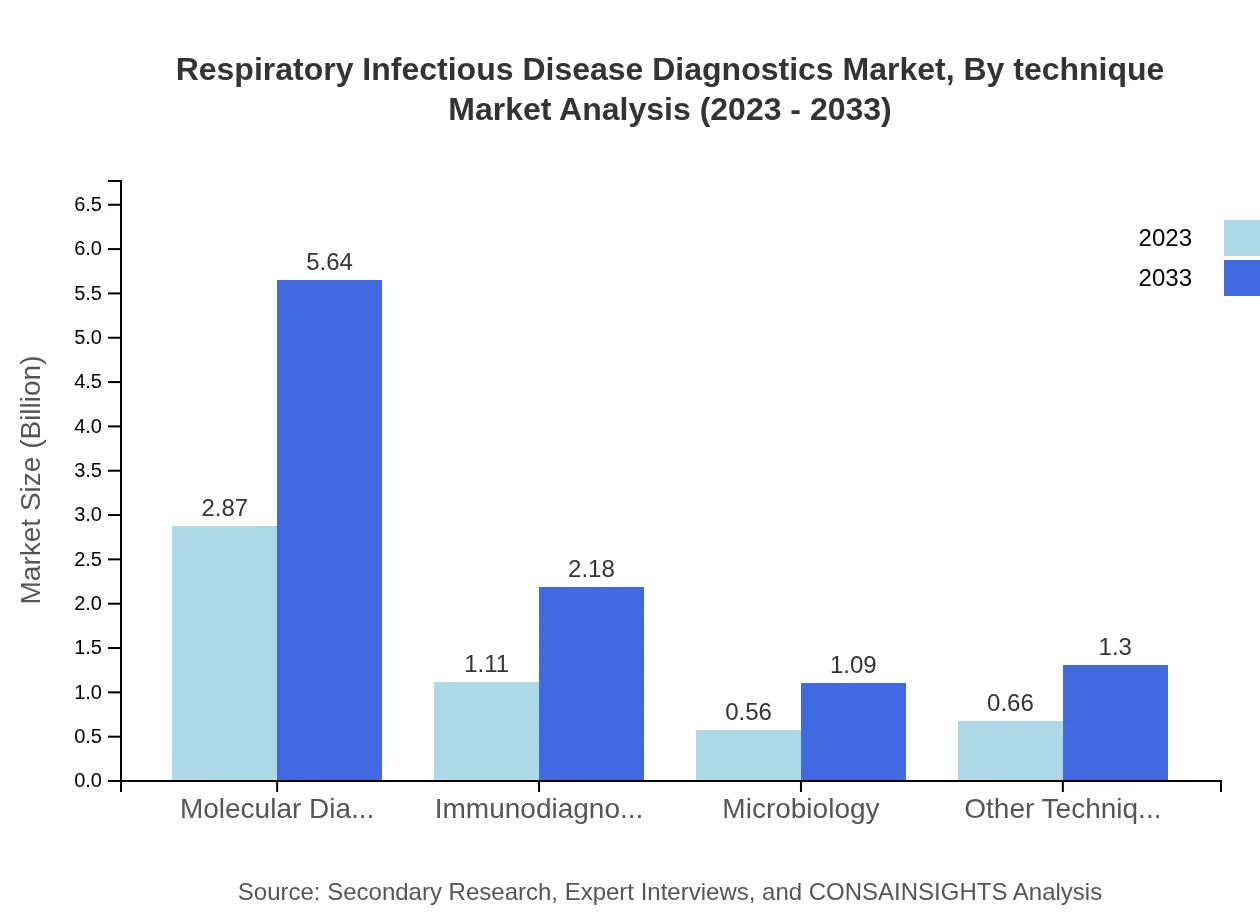
Respiratory Infectious Disease Diagnostics Market Analysis By Technique
Techniques including molecular diagnostics are set to dominate the market, anticipated to increase from $2.87 billion to $5.64 billion by 2033. This segment highlights the shift towards precision in diagnostics.
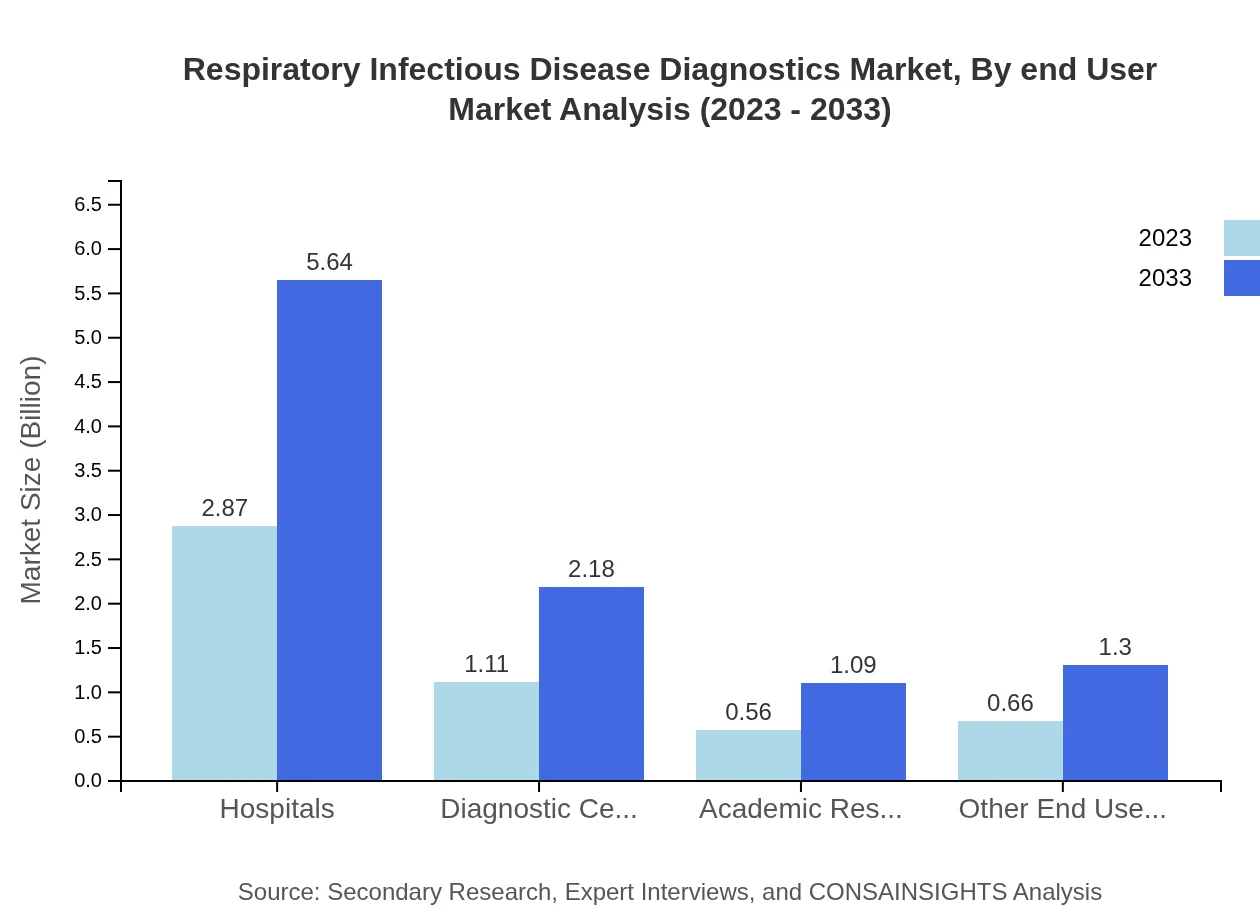
Respiratory Infectious Disease Diagnostics Market Analysis By End User
The end-user segment shows hospitals as leading users, with market sizes expanding from $2.87 billion to $5.64 billion by 2033. This trend reflects the necessity for advanced diagnostics in inpatient care.
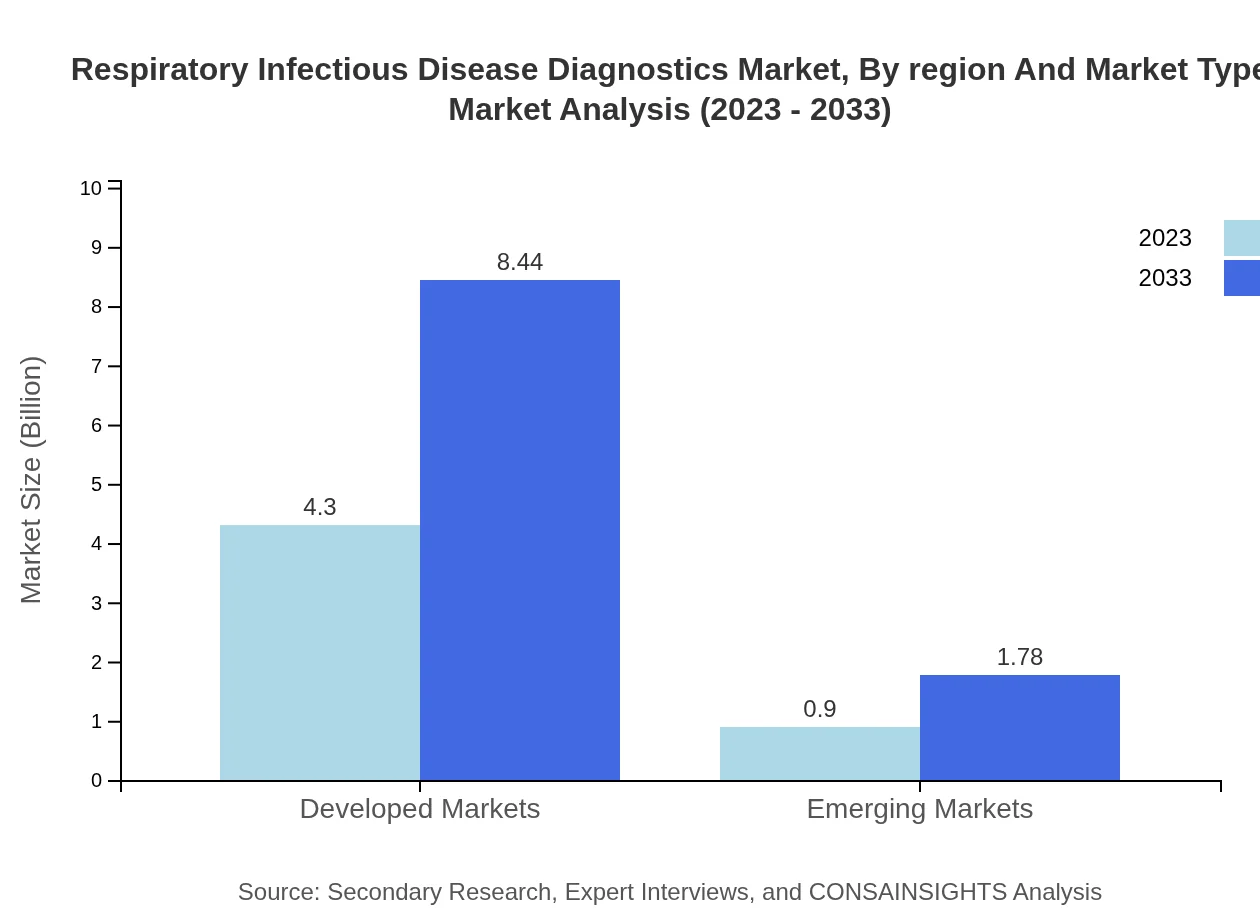
Respiratory Infectious Disease Diagnostics Market Analysis By Region And Market Type
This segment helps to visualize the market across diverse regions, emphasizing the pivotal role that developed markets play in overall revenue generation, as they contribute to over 82% by 2033.
Respiratory Infectious Disease Diagnostics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Respiratory Infectious Disease Diagnostics Industry
Thermo Fisher Scientific:
A leading provider of scientific instruments, reagents, and consumables, Thermo Fisher aims to deliver comprehensive diagnostic solutions, enhancing care quality in respiratory infectious diseases.Roche Diagnostics:
As a pioneer in healthcare, Roche Diagnostics focuses on innovative diagnostics in various fields, including respiratory diseases, investing in cutting-edge technologies.Siemens Healthineers:
Known for its advanced medical imaging and diagnostic solutions, Siemens Healthineers is committed to transforming the future of healthcare through innovative diagnostic tools.Abbott Laboratories:
Abbott is recognized for its extensive range of diagnostics, including advanced platforms for respiratory infectious diseases, focusing on rapid and accurate test solutions.BD (Becton, Dickinson and Company):
BD specializes in medical technology, providing innovative diagnostic tools, particularly in infectious diseases, enhancing patient outcomes through effective diagnostic practices.We're grateful to work with incredible clients.









FAQs
What is the market size of Respiratory Infectious Disease Diagnostics?
The global market for Respiratory Infectious Disease Diagnostics was valued at approximately $5.2 billion in 2023 and is projected to grow at a CAGR of 6.8% over the next decade, indicating a robust expansion in the industry.
What are the key market players or companies in this Respiratory Infectious Disease Diagnostics industry?
Key market players in the Respiratory Infectious Disease Diagnostics industry include major companies like Abbott Laboratories, Roche, Siemens Healthineers, BioMérieux, and Thermo Fisher Scientific, all of whom are instrumental in providing innovative diagnostic solutions.
What are the primary factors driving the growth in the Respiratory Infectious Disease Diagnostics industry?
Growth in the Respiratory Infectious Disease Diagnostics sector is primarily driven by increasing prevalence of respiratory diseases, advancements in diagnostic technologies, rising healthcare expenditures, and growing awareness surrounding early disease detection.
Which region is the fastest Growing in the Respiratory Infectious Disease Diagnostics market?
The fastest-growing region in the Respiratory Infectious Disease Diagnostics market is North America, with a market size projected to rise from $2.02 billion in 2023 to $3.97 billion by 2033, showcasing strong growth potential.
Does Consainsights provide customized market report data for the Respiratory Infectious Disease Diagnostics industry?
Yes, Consainsights offers customized market report data for the Respiratory Infectious Disease Diagnostics industry, allowing clients to obtain specific insights tailored to their needs and objectives.
What deliverables can I expect from this Respiratory Infectious Disease Diagnostics market research project?
Deliverables from the Respiratory Infectious Disease Diagnostics market research project typically include comprehensive market analysis reports, segmentation data, trend forecasts, competitive landscape insights, and strategic recommendations.
What are the market trends of Respiratory Infectious Disease Diagnostics?
Key trends in the Respiratory Infectious Disease Diagnostics market include the rise of molecular diagnostics, increasing utilization of point-of-care testing, innovative diagnostic methods, and the expansion of telemedicine solutions.