Resuscitation Devices Market Report
Published Date: 31 January 2026 | Report Code: resuscitation-devices
Resuscitation Devices Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Resuscitation Devices market, covering key insights on market size, trends, segmentation, regional analysis, and forecasts from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
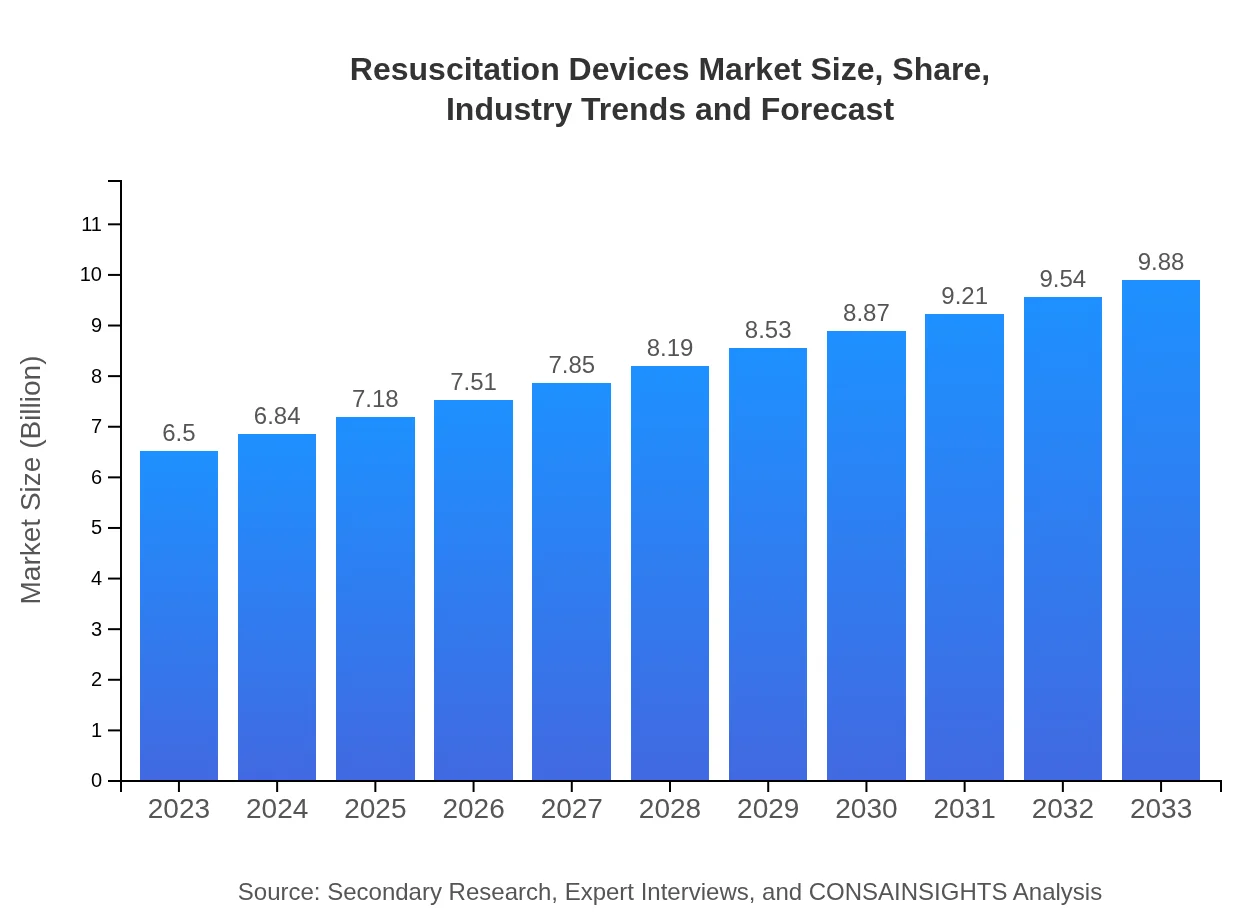
| 2023 Market Size | $6.50 Billion |
| CAGR (2023-2033) | 4.2% |
| 2033 Market Size | $9.88 Billion |
| Top Companies | Medtronic , Philips Healthcare, ZOLL Medical Corporation, Stryker Corporation |
| Last Modified Date | 31 January 2026 |
Resuscitation Devices Market Overview
Customize Resuscitation Devices Market Report market research report
- ✔ Get in-depth analysis of Resuscitation Devices market size, growth, and forecasts.
- ✔ Understand Resuscitation Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Resuscitation Devices
What is the Market Size & CAGR of Resuscitation Devices market in 2023?
Resuscitation Devices Industry Analysis
Resuscitation Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
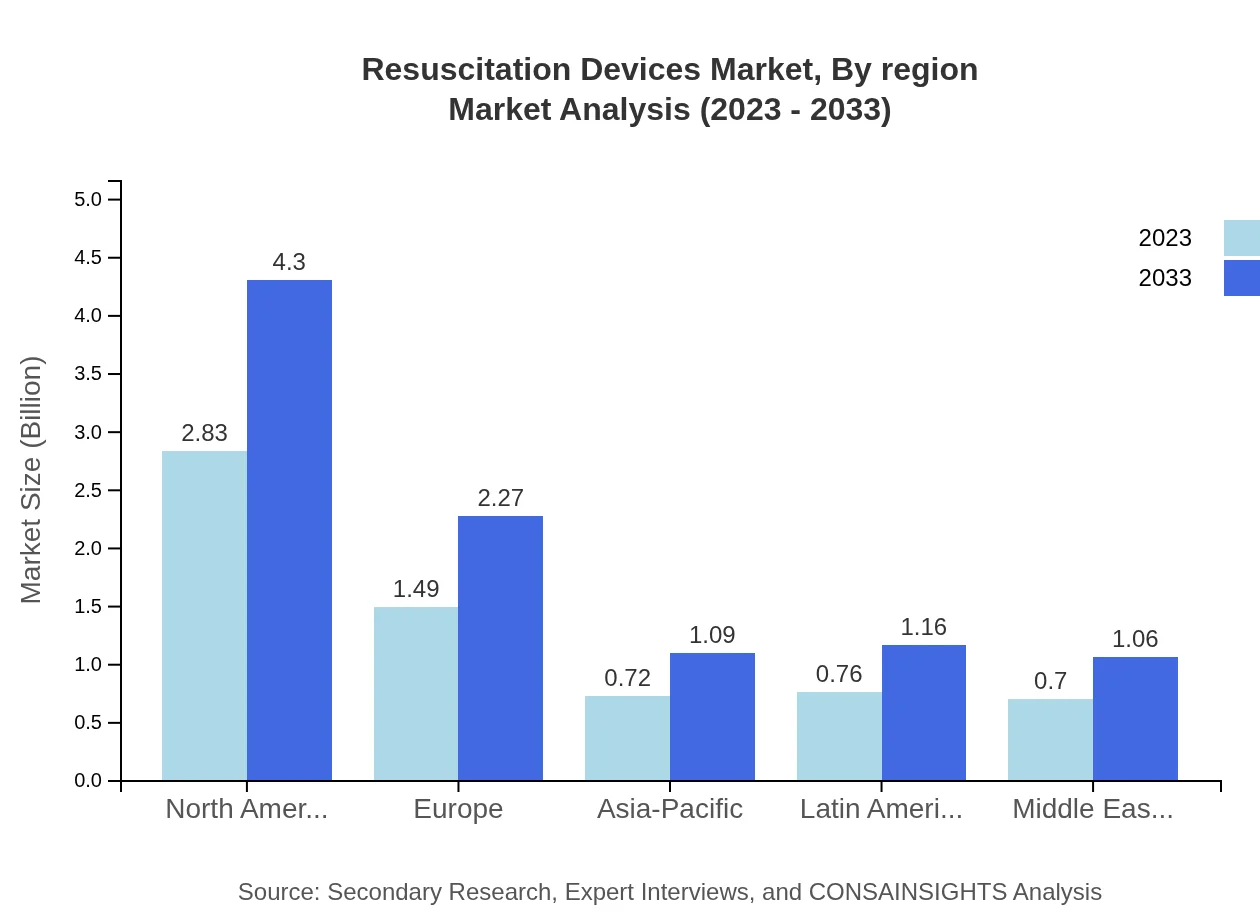
Resuscitation Devices Market Analysis Report by Region
Europe Resuscitation Devices Market Report:
The European market is projected to grow from $1.99 billion in 2023 to $3.02 billion by 2033. The region benefits from a strong regulatory framework and high investment in medical technologies, bolstering the adoption of innovative resuscitation devices.Asia Pacific Resuscitation Devices Market Report:
The Asia-Pacific region is expected to witness significant growth, with a market size projected to increase from $1.11 billion in 2023 to $1.69 billion in 2033. The rapid expansion of healthcare infrastructure and rising awareness of emergency medical services drive the demand for resuscitation devices in this region, particularly in countries like China and India.North America Resuscitation Devices Market Report:
North America holds the largest share of the resuscitation devices market, valued at approximately $2.43 billion in 2023 and expected to grow to $3.70 billion by 2033. This growth is fueled by advanced healthcare systems, high awareness of cardiac emergencies, and technological advancements in resuscitation methodologies.South America Resuscitation Devices Market Report:
In South America, the market is anticipated to grow from $0.64 billion in 2023 to $0.97 billion by 2033, supported by increasing healthcare expenditures and initiatives aimed at improving emergency response systems.Middle East & Africa Resuscitation Devices Market Report:
In the Middle East and Africa, the market is expected to rise from $0.33 billion in 2023 to $0.50 billion by 2033. Growth in this region is driven by improvements in healthcare accessibility and emergency medical service capabilities.Tell us your focus area and get a customized research report.
Resuscitation Devices Market Analysis By Device Type
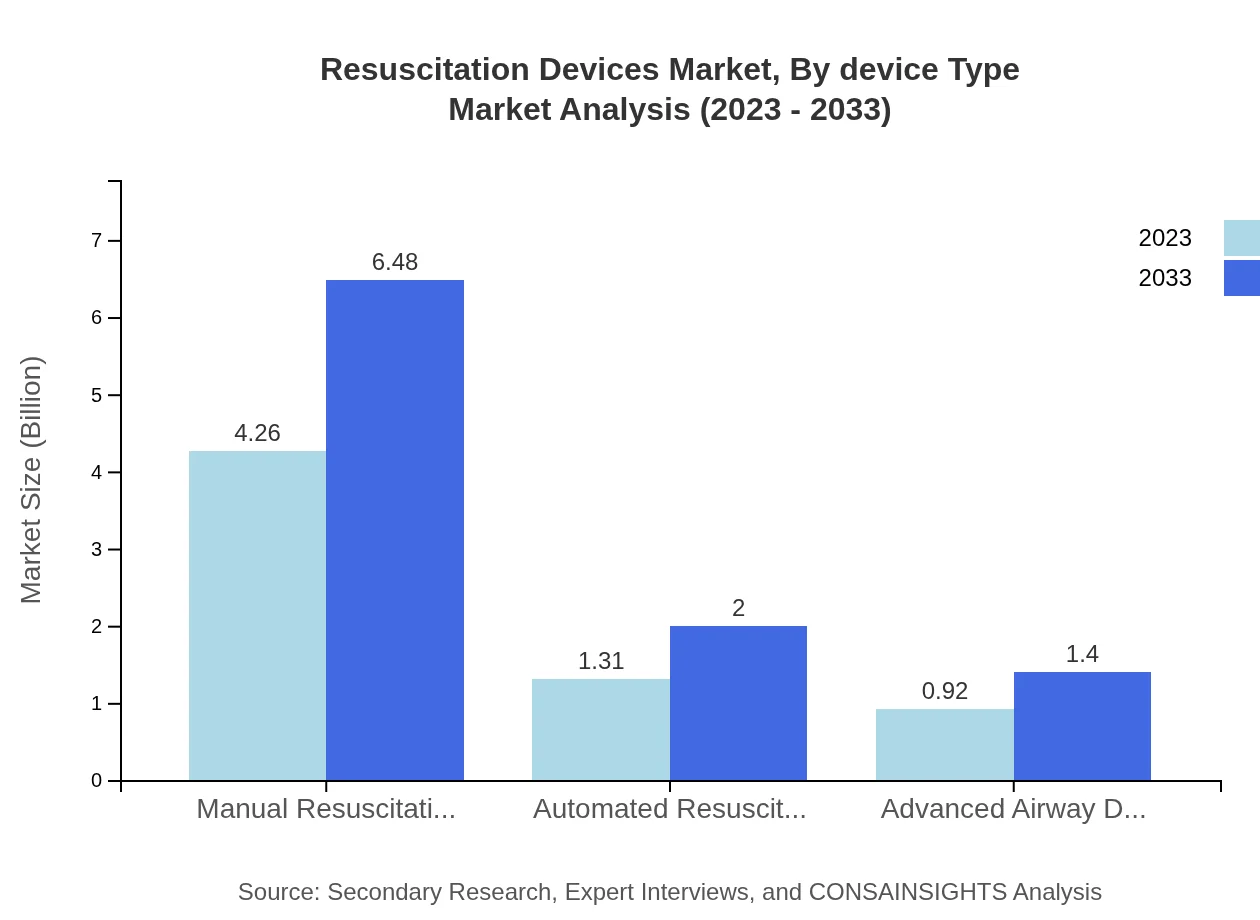
The device type segment of the Resuscitation Devices market includes Manual Resuscitation Devices, Automated Resuscitation Devices, and Advanced Airway Devices. As of 2023, manual resuscitation devices hold a significant market share of 65.61%, projected to reach a valuation of $6.48 billion by 2033. Automated devices, representing 20.19% share, are gaining traction due to their ease of use and efficiency, with expected growth to $2.00 billion. Advanced airway devices, comprising 14.2%, are also expected to witness sustained growth, reaching $1.40 billion.
Resuscitation Devices Market Analysis By Application
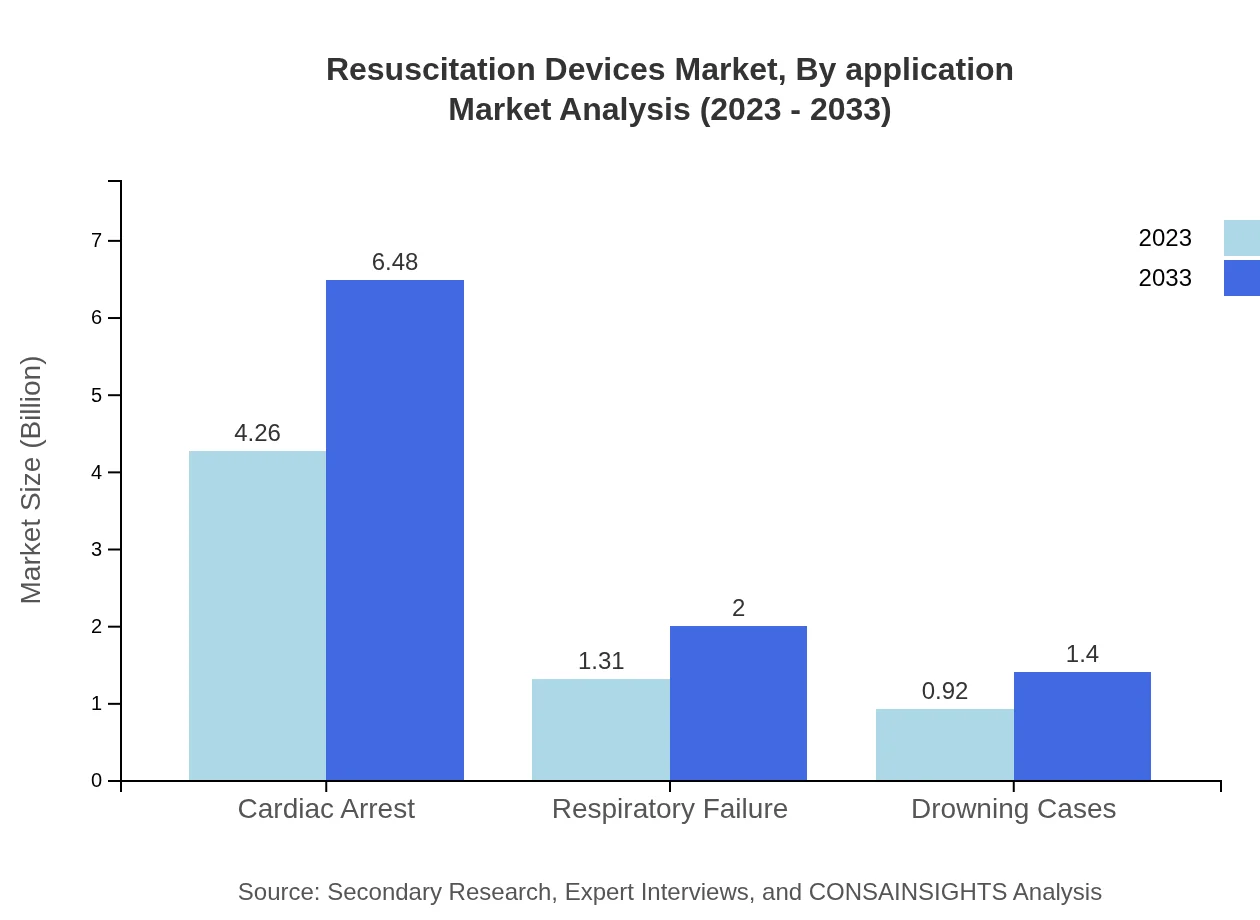
The application segment includes Cardiac Arrest, Respiratory Failure, and Drowning Cases. Cardiac arrest applications dominate the market, expected to maintain a share of 65.61%, growing to $6.48 billion by 2033. Respiratory failure applications account for 20.19% of the market, projected to reach $2.00 billion. Drowning cases represent a smaller share of 14.2%, with growth to $1.40 billion as awareness and treatment options improve.
Resuscitation Devices Market Analysis By End User
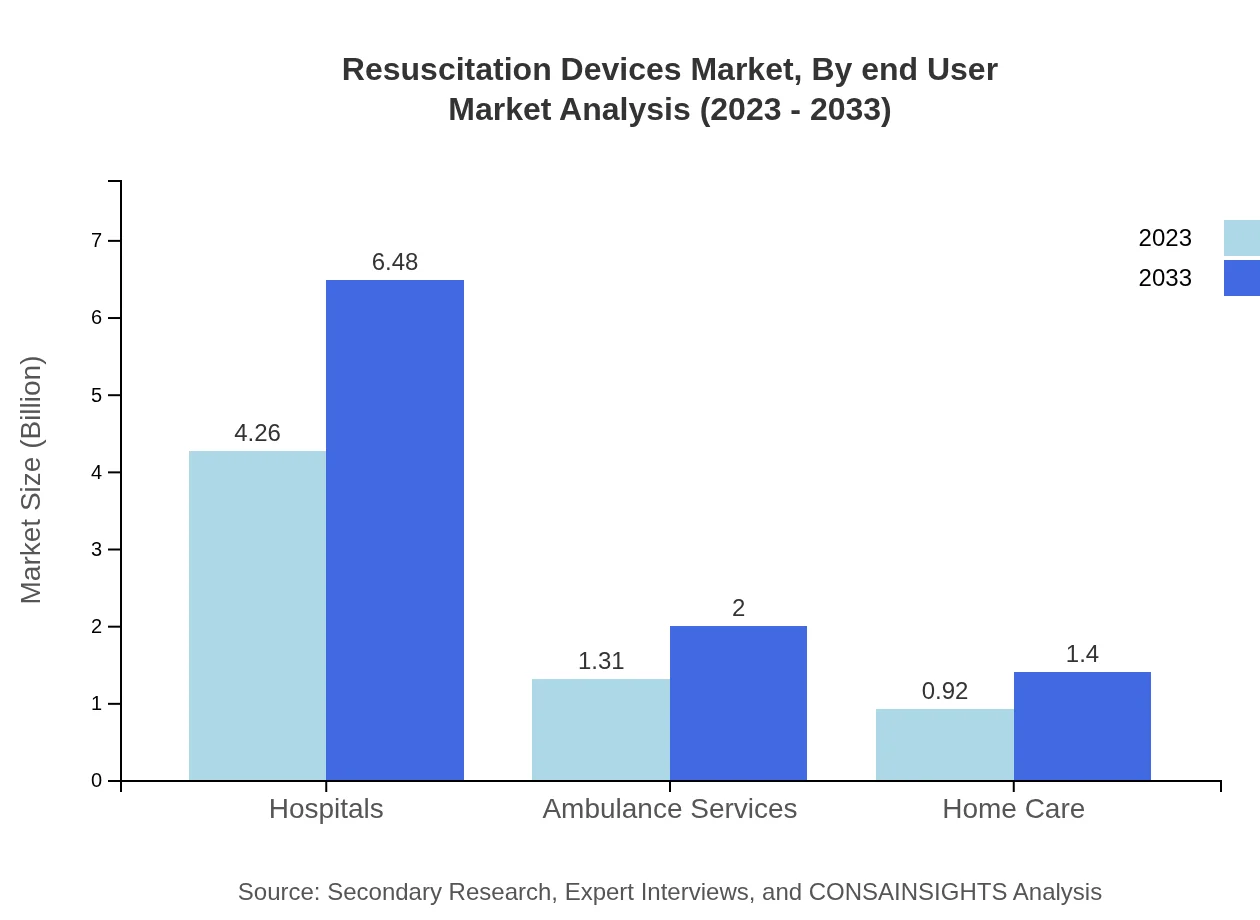
In terms of end-users, Hospitals dominate, with a significant share of 65.61% in 2023, translating to $6.48 billion by 2033. Ambulance services hold a share of 20.19%, expected to grow to $2.00 billion. Home care accounts for 14.2% of the market, projected to reach $1.40 billion, emphasizing the shift toward patient management at home.
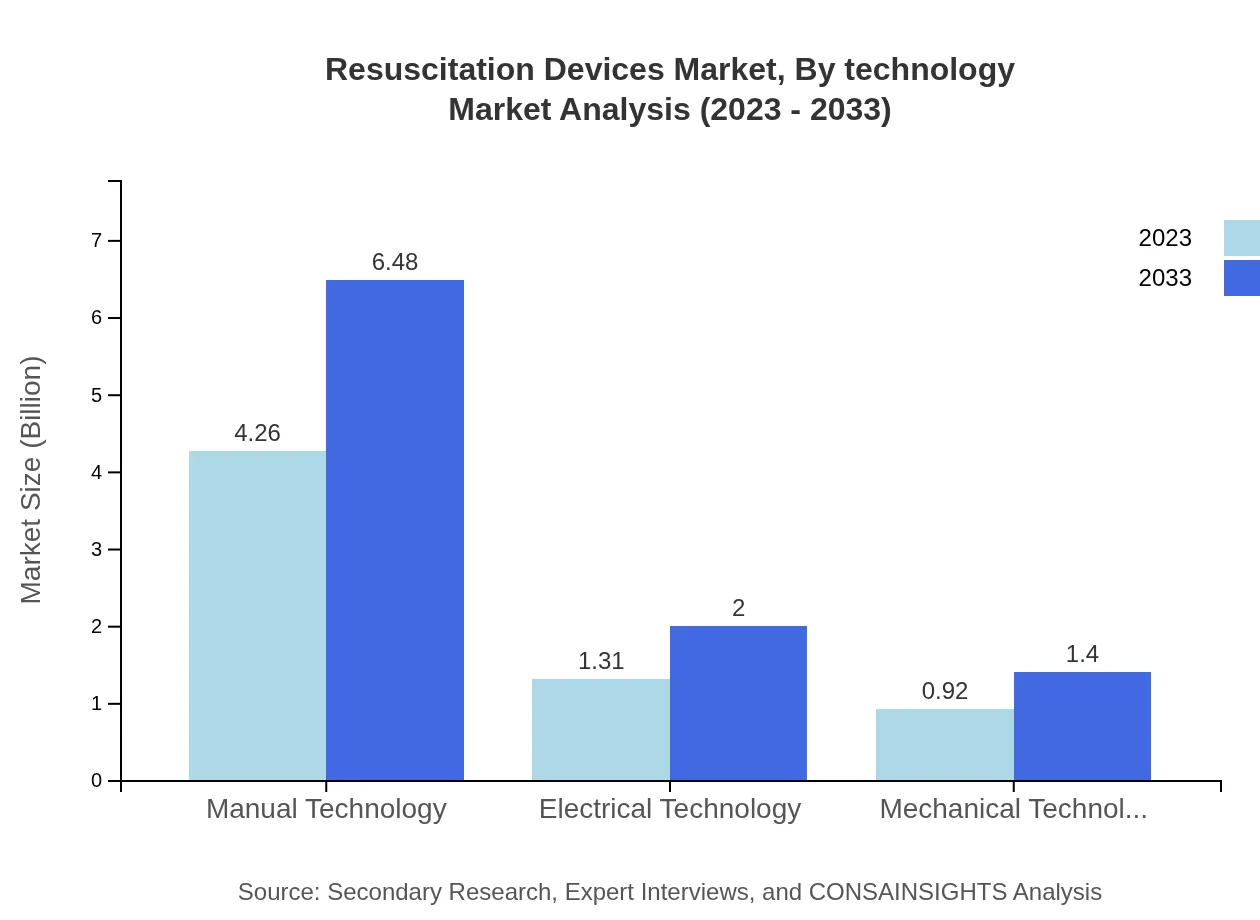
Resuscitation Devices Market Analysis By Technology
The technology segment is classified into Manual Technology, Electrical Technology, and Mechanical Technology. Manual technology holds the largest market share of 65.61%, with a growth trajectory towards $6.48 billion. Electrical technology stands at 20.19%, with a forecast to reach $2.00 billion, while mechanical technology accounts for 14.2%, expected to reach $1.40 billion as innovations enhance device functionality.
Resuscitation Devices Market Analysis By Region
A regional analysis highlights the discrepancies in market growth and acceptance trends. North America leads significantly, followed by Europe and Asia-Pacific. The shared growth potential in emerging regions such as the Middle East and Africa, alongside the robust healthcare frameworks in developed regions, indicate a balanced outlook for market expansion.
Resuscitation Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Resuscitation Devices Industry
Medtronic :
A key player in developing advanced resuscitation devices, Medtronic specializes in innovative cardiovascular and monitoring services, significantly impacting emergency care.Philips Healthcare:
Philips is known for its advanced cardiopulmonary resuscitation solutions, offering a range of automated devices that are widely used in hospitals and emergency services.ZOLL Medical Corporation:
ZOLL produces life-saving devices and software solutions in emergency medicine, contributing to advancements in defibrillation and post-resuscitation care.Stryker Corporation:
Stryker's medical technologies include specialized resuscitation devices, such as automatic defibrillators, that are integral to modern emergency response efforts.We're grateful to work with incredible clients.









FAQs
What is the market size of resuscitation devices?
The global resuscitation devices market is projected to reach approximately $6.5 billion by 2033, growing at a CAGR of 4.2%. This growth reflects increased demand for advanced medical equipment and enhanced emergency care services.
What are the key market players or companies in this resuscitation devices industry?
Key players in the resuscitation devices market include major healthcare companies and manufacturers known for innovative life-saving technologies, contributing significantly to market development through R&D investments and strategic partnerships.
What are the primary factors driving the growth in the resuscitation devices industry?
Growth drivers in the resuscitation devices market include technological advancements, increasing prevalence of cardiac diseases, rising demand for emergency services, awareness campaigns on CPR, and government investments in healthcare infrastructure.
Which region is the fastest Growing in the resuscitation devices market?
Asia-Pacific is currently the fastest-growing region in the resuscitation devices market, with projected growth from $1.11 billion in 2023 to $1.69 billion in 2033, indicating a strong market potential due to healthcare development initiatives.
Does ConsaInsights provide customized market report data for the resuscitation devices industry?
Yes, Consainsights offers customized market report data tailored to specific needs in the resuscitation devices industry, ensuring clients receive relevant insights for strategic planning and market positioning.
What deliverables can I expect from this resuscitation devices market research project?
Deliverables from the resuscitation devices market research project include comprehensive market analysis reports, insights on regional segments, competitor comparisons, and trends affecting market dynamics, providing valuable data for decision-making.
What are the market trends of resuscitation devices?
Current trends in the resuscitation devices market focus on the development of automated devices, integration of telemedicine, increasing emphasis on training programs, and the growing use of mobile medical technologies to enhance patient outcomes.