Retinal Biologics Market Report
Published Date: 31 January 2026 | Report Code: retinal-biologics
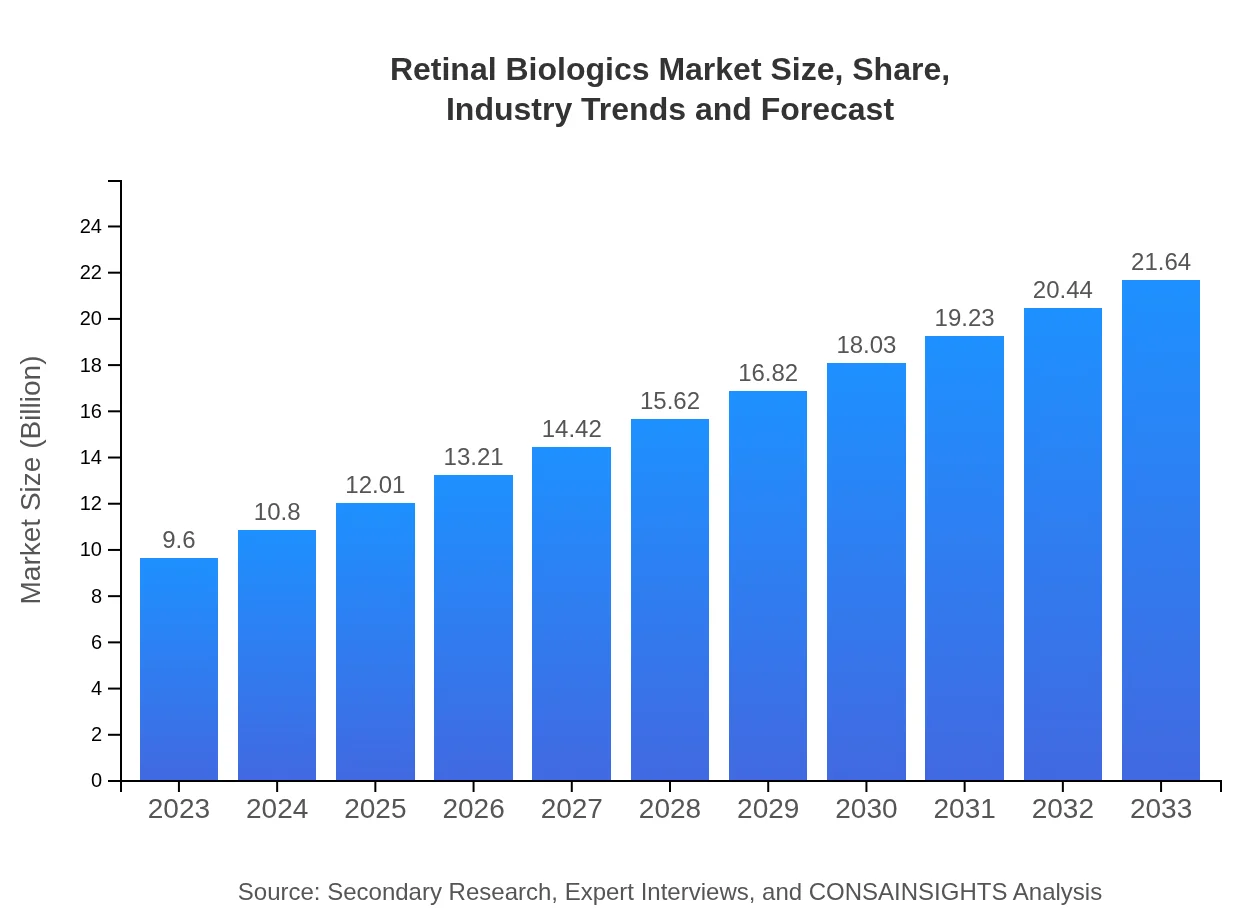
Retinal Biologics Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Retinal Biologics market from 2023 to 2033, examining market size, growth potential, trends, and competitive dynamics. Additionally, it offers insights into segmentation, regional performance, and leading companies within the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $9.60 Billion |
| CAGR (2023-2033) | 8.2% |
| 2033 Market Size | $21.64 Billion |
| Top Companies | Regeneron Pharmaceuticals, Inc., Novartis AG, Roche Holding AG, Bayer AG |
| Last Modified Date | 31 January 2026 |
Retinal Biologics Market Overview
Customize Retinal Biologics Market Report market research report
- ✔ Get in-depth analysis of Retinal Biologics market size, growth, and forecasts.
- ✔ Understand Retinal Biologics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Retinal Biologics
What is the Market Size & CAGR of Retinal Biologics market in 2023?
Retinal Biologics Industry Analysis
Retinal Biologics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Retinal Biologics Market Analysis Report by Region
Europe Retinal Biologics Market Report:
The European market is projected to increase from USD 2.74 billion in 2023 to USD 6.19 billion by 2033. Enhanced regulatory frameworks and strong healthcare systems are vital components, allowing for timely access to innovative retinal treatments.Asia Pacific Retinal Biologics Market Report:
In the Asia Pacific region, the market is expected to grow from USD 1.85 billion in 2023 to USD 4.16 billion by 2033. Factors driving this growth include increasing healthcare accessibility, a rising geriatric population susceptible to retinal diseases, and investments in healthcare infrastructure.North America Retinal Biologics Market Report:
North America is expected to dominate the market, with growth from USD 3.33 billion in 2023 to USD 7.51 billion by 2033. The presence of leading healthcare providers, innovative research initiatives, and favorable reimbursement policies contribute significantly to this growth.South America Retinal Biologics Market Report:
The South American market, with a growth trajectory from USD 0.53 billion in 2023 to USD 1.21 billion by 2033, is anticipated to expand due to increased awareness of retinal diseases and improvements in healthcare facilities, though challenges like economic fluctuations may impact growth.Middle East & Africa Retinal Biologics Market Report:
In the Middle East and Africa, the market is expected to grow from USD 1.14 billion in 2023 to USD 2.58 billion by 2033, spurred on by initiatives to improve eye care and expand access to biologic therapies.Tell us your focus area and get a customized research report.
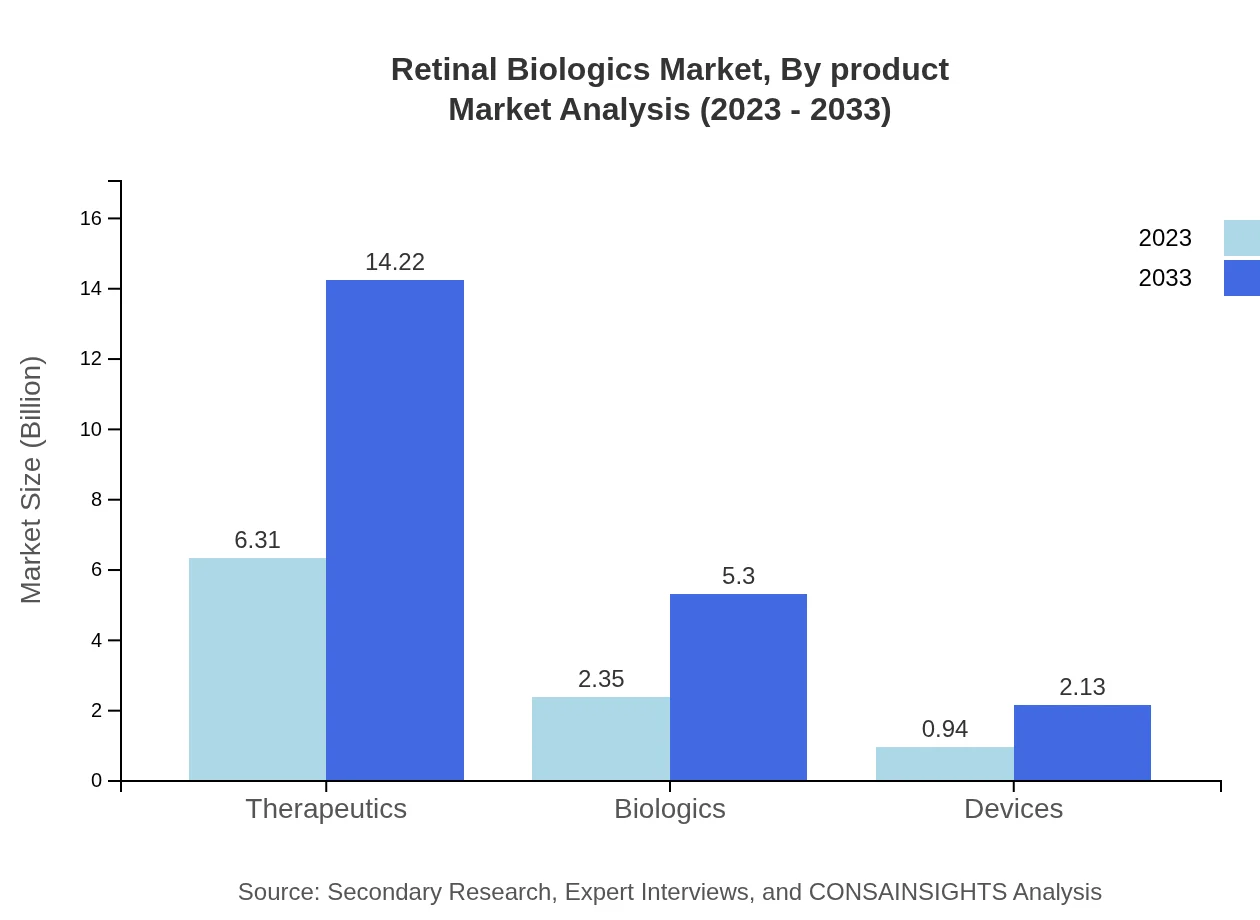
Retinal Biologics Market Analysis By Product
The product segment of the Retinal Biologics market is dominated by therapeutics, valued at USD 6.31 billion in 2023, and projected to reach USD 14.22 billion by 2033. Biologics follow, starting at USD 2.35 billion and estimably growing to USD 5.30 billion. These products are critical in addressing the widespread conditions affecting the retina, with therapeutics capturing the majority share due to their established efficacy and clinical use.
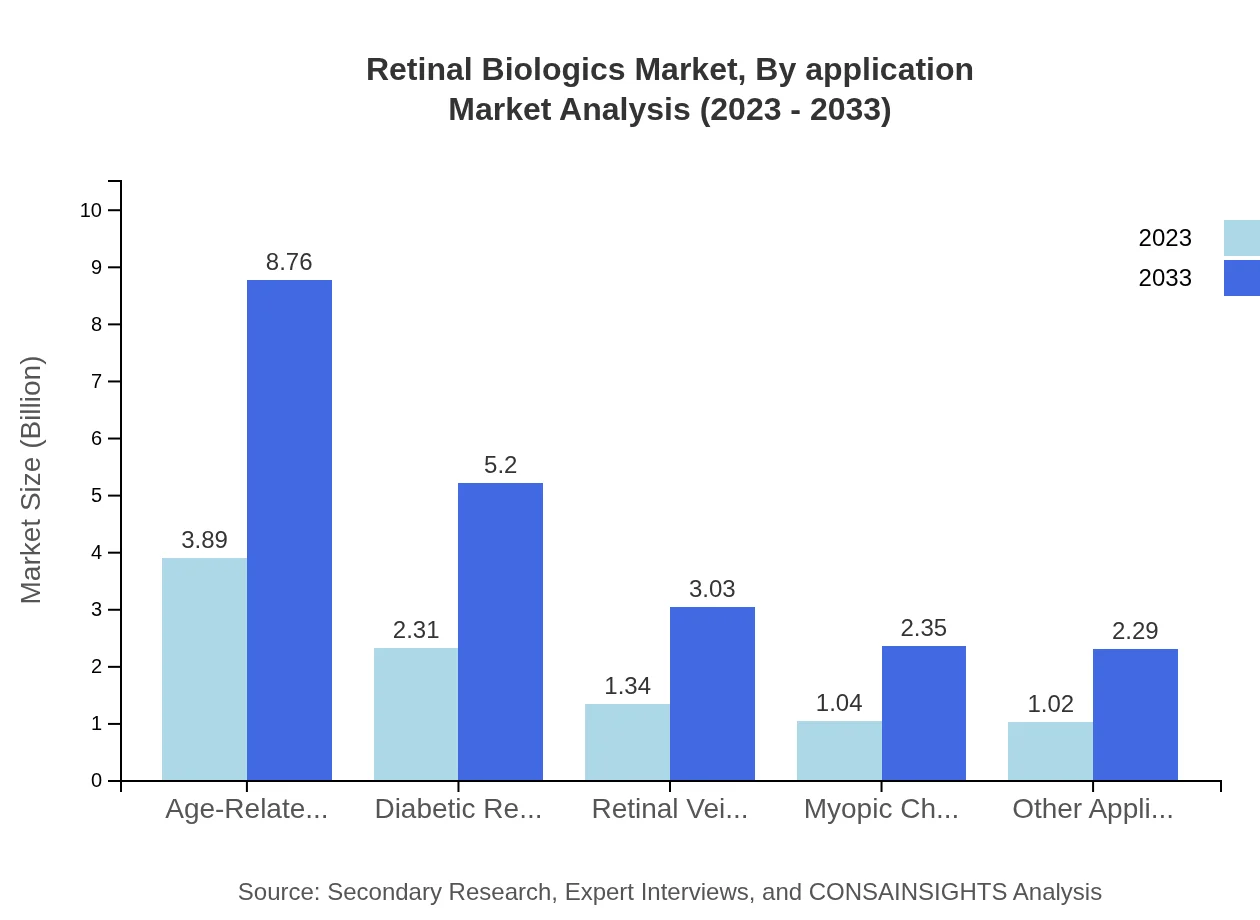
Retinal Biologics Market Analysis By Application
The leading application in the market is for age-related macular degeneration, projected to continue growing from USD 3.89 billion in 2023 to USD 8.76 billion by 2033. Diabetic retinopathy and retinal vein occlusion follow, sharing a substantial market presence, reflecting the significant healthcare burden these conditions represent.
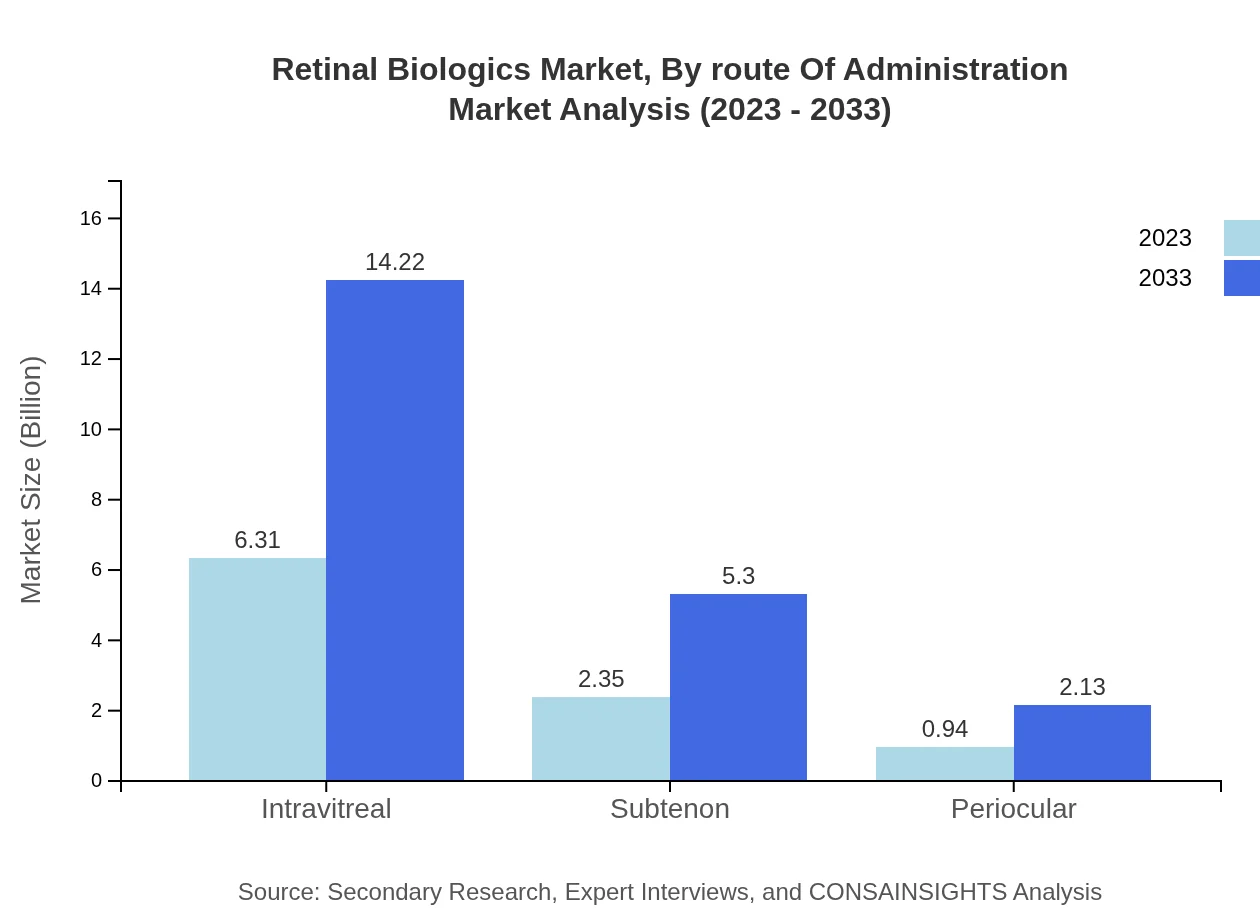
Retinal Biologics Market Analysis By Route Of Administration
The intravitreal route of administration leads the market, expected to increase from USD 6.31 billion in 2023 to USD 14.22 billion by 2033. This method is preferred due to its direct targeting of retinal tissues, maximizing therapeutic benefits.
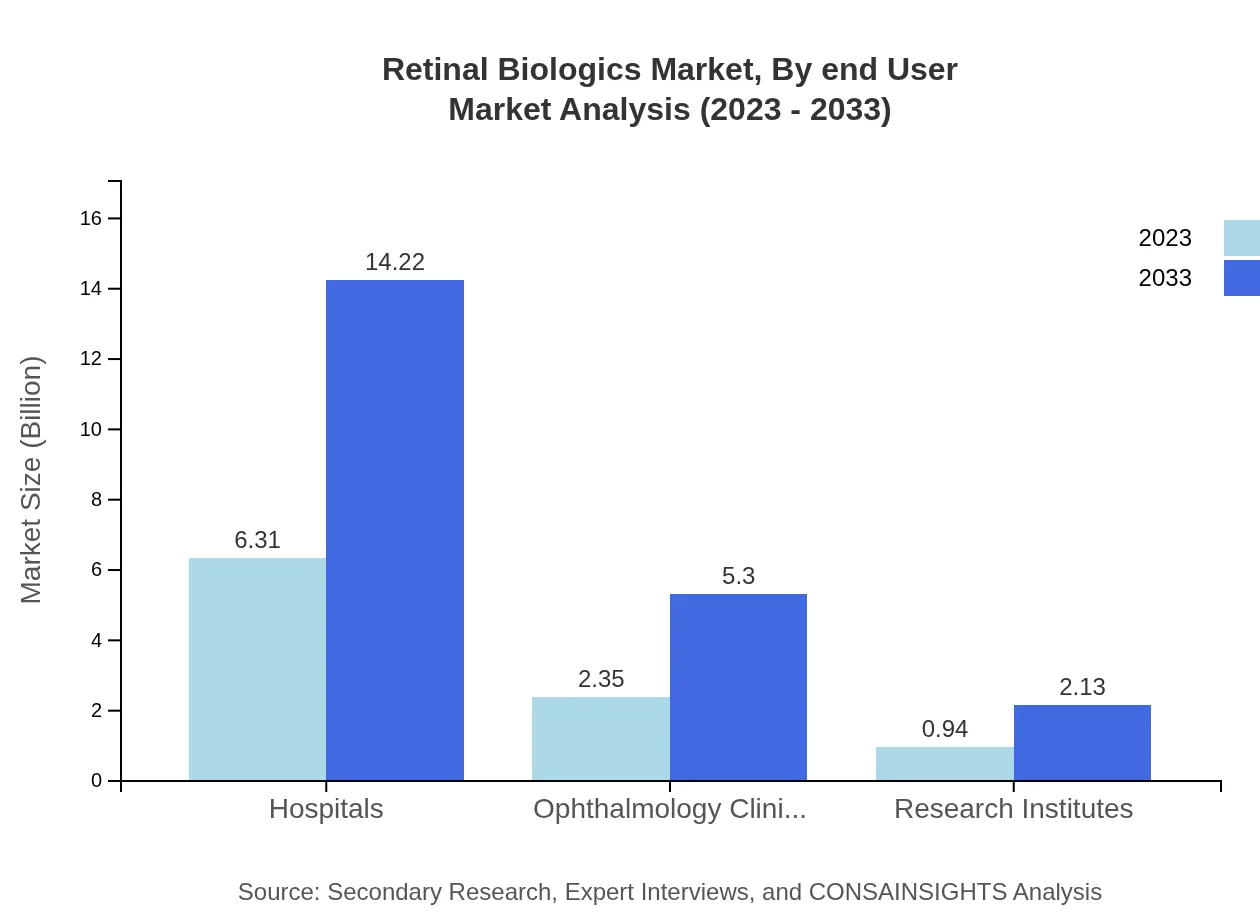
Retinal Biologics Market Analysis By End User
Hospitals remain the primary end-users, accounting for sales worth USD 6.31 billion in 2023, anticipated to grow to USD 14.22 billion by 2033. Ophthalmology clinics and research institutes follow, with increasing numbers of specialized institutions focusing on retinal health.
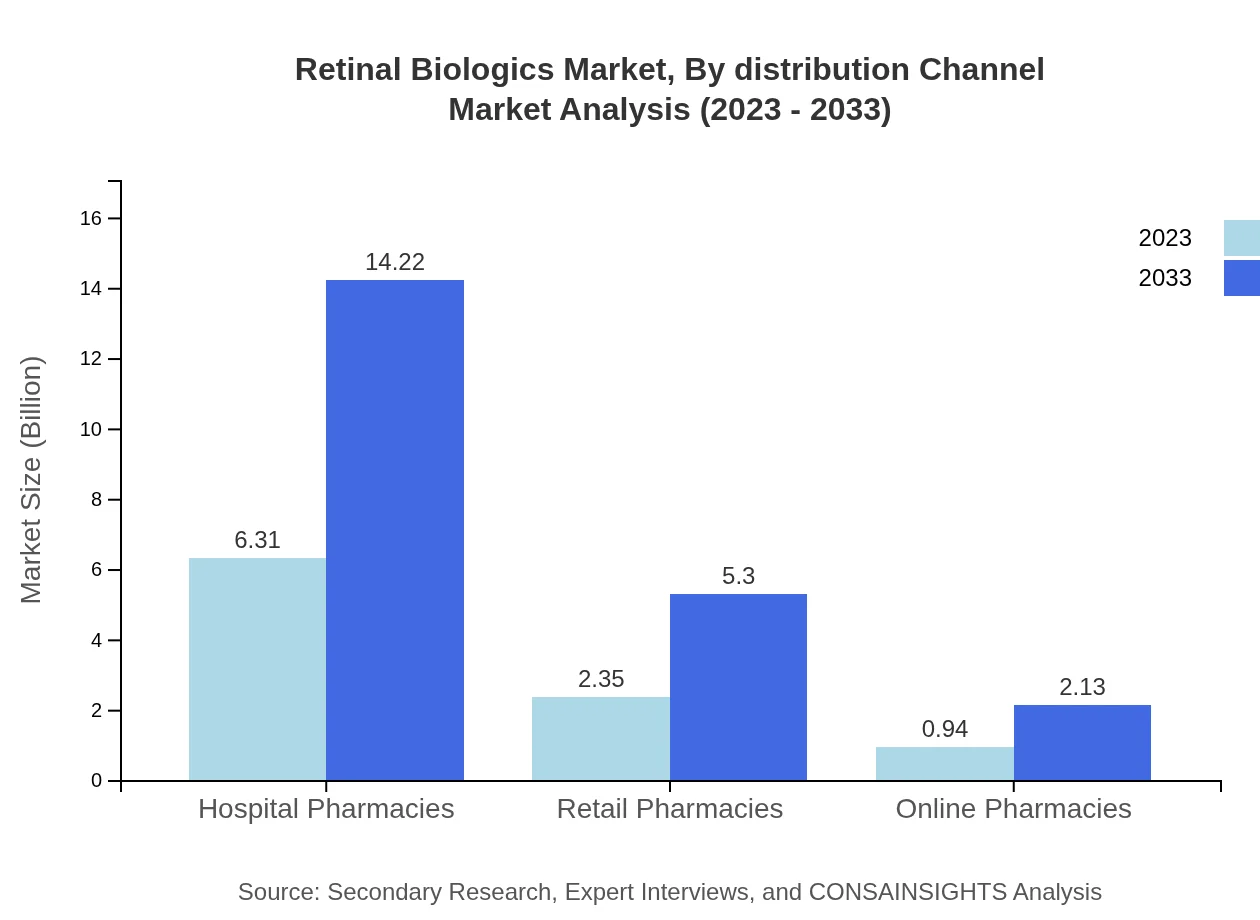
Retinal Biologics Market Analysis By Distribution Channel
Hospital pharmacies are the primary distribution channel, holding a market size of USD 6.31 billion in 2023 and projected to rise to USD 14.22 billion by 2033. Retail pharmacies and online pharmacies also play growing roles in reaching patients more effectively.
Retinal Biologics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Retinal Biologics Industry
Regeneron Pharmaceuticals, Inc.:
A leader in the development of medical treatments for retinal diseases, known for its innovation in developing therapies such as Eylea.Novartis AG:
This global healthcare company focuses on novel therapies for eye diseases, with a significant portfolio addressing various retinal conditions.Roche Holding AG:
Acclaimed for its research-driven approach, Roche develops targeted biologics for retinal diseases, contributing substantially to the industry.Bayer AG:
Bayer is recognized for its advancements in retinal therapies and its commitment to expanding treatment options available to patients.We're grateful to work with incredible clients.









FAQs
What is the market size of retinal Biologics?
The global retinal biologics market is currently valued at approximately $9.6 billion and is projected to grow at a CAGR of 8.2% from 2023 to 2033, highlighting significant growth opportunities within the industry.
What are the key market players or companies in this retinal Biologics industry?
The retinal biologics industry features major players such as Genentech, Regeneron Pharmaceuticals, and Novartis, which innovate and supply therapies for retinal diseases, ensuring robust market competition and advancements in treatment options.
What are the primary factors driving the growth in the retinal Biologics industry?
Key growth factors for the retinal biologics market include an increasing prevalence of retinal diseases, advancements in biologics technology, and a growing aging population that necessitates innovative therapeutic solutions.
Which region is the fastest Growing in the retinal Biologics?
The Asia Pacific region is the fastest-growing market for retinal biologics, with growth projected from $1.85 billion in 2023 to $4.16 billion by 2033, driven by rising healthcare accessibility and demand for innovative therapies.
Does Consainsights provide customized market report data for the retinal Biologics industry?
Yes, Consainsights offers customized market reports tailored to specific needs within the retinal biologics industry, ensuring clients receive relevant and actionable insights aligned with their business strategies.
What deliverables can I expect from this retinal Biologics market research project?
Deliverables typically include comprehensive market analysis reports, segment data analysis, growth forecasts, and insights on competitive landscape, enabling informed decision-making for stakeholders in retinal biologics.
What are the market trends of retinal Biologics?
Current trends in the retinal biologics market include a surge in personalized medicine approaches, increased investment in research, and the development of innovative delivery methods aiming to improve patient outcomes.