Retinoblastoma Treatment Market Report
Published Date: 31 January 2026 | Report Code: retinoblastoma-treatment
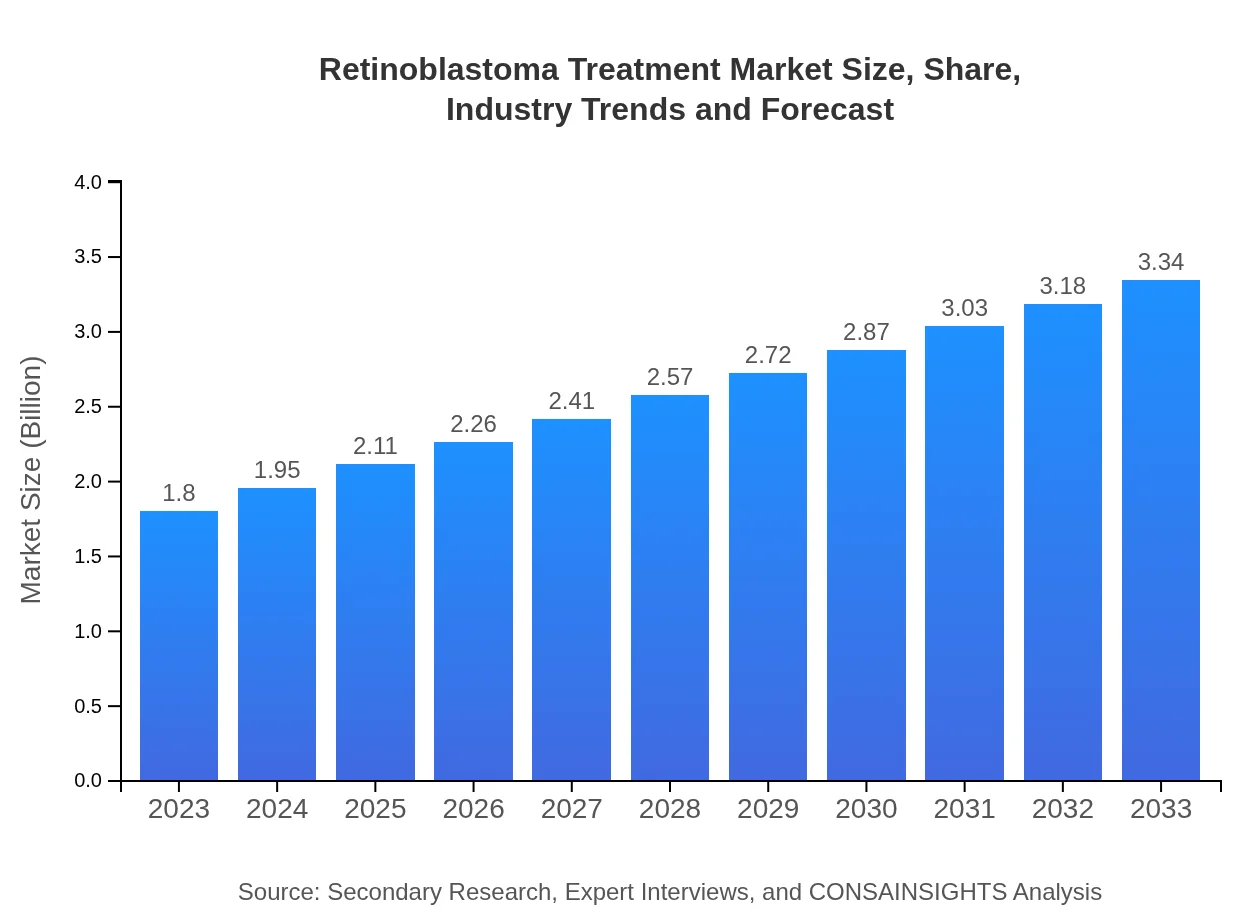
Retinoblastoma Treatment Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Retinoblastoma Treatment market, offering insights into market trends, segmentation, regional performance, and forecasts from 2023 to 2033. The report aims to equip stakeholders with valuable data to make informed strategic decisions.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $1.80 Billion |
| CAGR (2023-2033) | 6.2% |
| 2033 Market Size | $3.34 Billion |
| Top Companies | Roche, Bristol-Myers Squibb, Novartis, Genentech |
| Last Modified Date | 31 January 2026 |
Retinoblastoma Treatment Market Overview
Customize Retinoblastoma Treatment Market Report market research report
- ✔ Get in-depth analysis of Retinoblastoma Treatment market size, growth, and forecasts.
- ✔ Understand Retinoblastoma Treatment's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Retinoblastoma Treatment
What is the Market Size & CAGR of Retinoblastoma Treatment market in 2023?
Retinoblastoma Treatment Industry Analysis
Retinoblastoma Treatment Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Retinoblastoma Treatment Market Analysis Report by Region
Europe Retinoblastoma Treatment Market Report:
Europe's market starts at USD 0.55 billion in 2023 and is anticipated to reach USD 1.01 billion by 2033. Strong regulatory frameworks and initiatives to improve early diagnosis and treatment adoption play pivotal roles in this region.Asia Pacific Retinoblastoma Treatment Market Report:
In the Asia Pacific, the market size in 2023 is estimated at USD 0.30 billion, projected to grow to USD 0.56 billion by 2033, driven by increasing healthcare expenditure and improved access to oncology services. Countries like India and China are seeing significant investments in pediatric healthcare.North America Retinoblastoma Treatment Market Report:
North America leads with a market size of USD 0.70 billion in 2023, projected to grow to USD 1.30 billion by 2033. The presence of advanced healthcare facilities and a high incidence of retinoblastoma contribute to this growth, alongside robust research and development activities.South America Retinoblastoma Treatment Market Report:
The South American market is smaller, with a size of USD 0.06 billion in 2023 and expected to reach USD 0.11 billion by 2033. The healthcare infrastructure is improving, yet challenges remain related to access and affordability of advanced treatments.Middle East & Africa Retinoblastoma Treatment Market Report:
The Middle East and Africa exhibit a market size of USD 0.20 billion in 2023 and is expected to grow to USD 0.36 billion by 2033. This region's market growth is hindered by limited access to advanced healthcare, but increasing investments are expected to facilitate better treatment options.Tell us your focus area and get a customized research report.
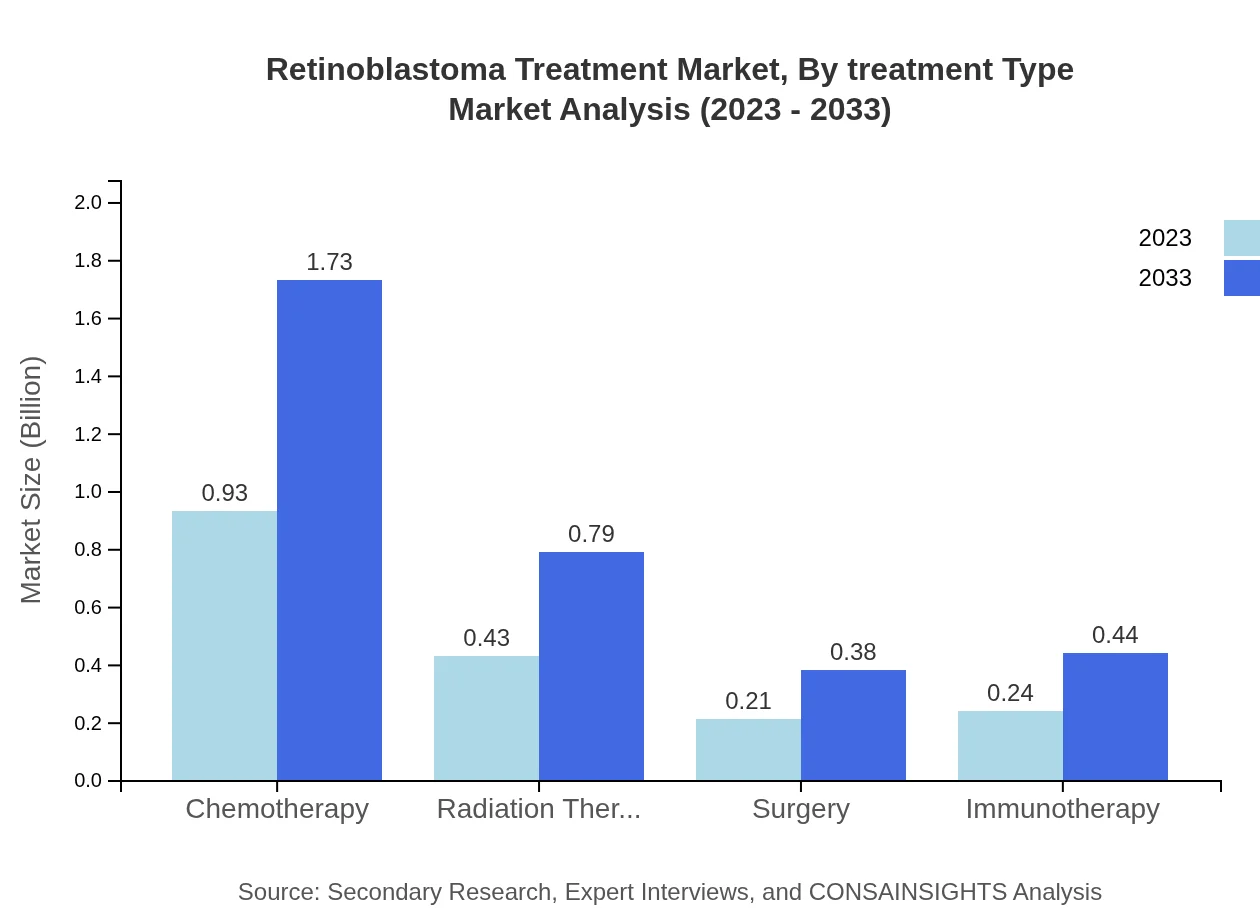
Retinoblastoma Treatment Market Analysis By Treatment Type
In 2023, the treatment type analysis reveals that chemotherapy holds a significant share with a market size of USD 0.93 billion, representing 51.75% of the total market. Radiation therapy and surgery follow, with market sizes of USD 0.43 billion and USD 0.21 billion, respectively. By 2033, chemotherapy is projected to grow to USD 1.73 billion, while radiation therapy and surgery are expected to reach USD 0.79 billion and USD 0.38 billion.
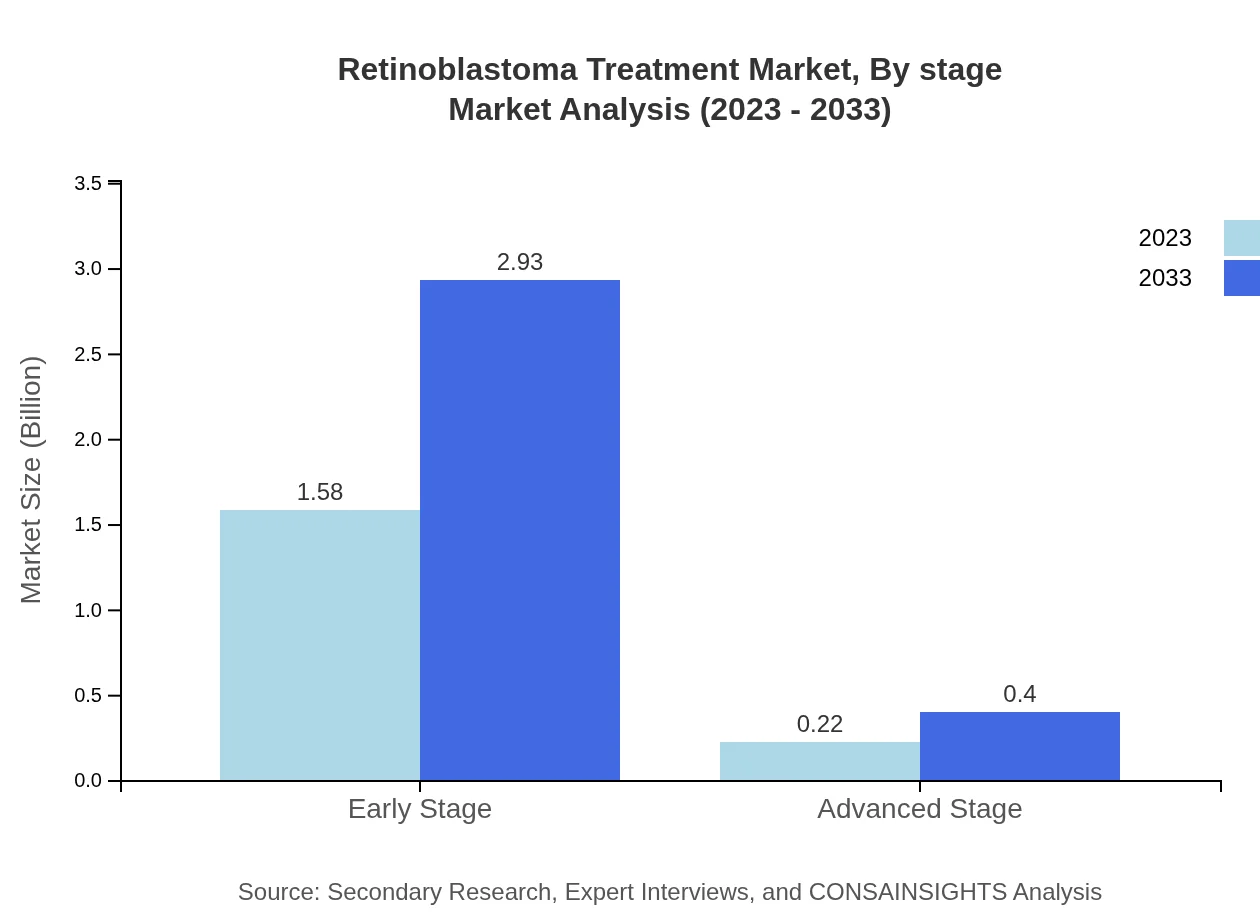
Retinoblastoma Treatment Market Analysis By Stage
The market segmentation by disease stage indicates that early-stage treatments dominate with a market size of USD 1.58 billion in 2023, equating to 87.93% share, and is forecasted to grow to USD 2.93 billion by 2033. Advanced-stage treatments account for 12.07% of the market with a size of USD 0.22 billion in 2023, expected to rise to USD 0.40 billion.
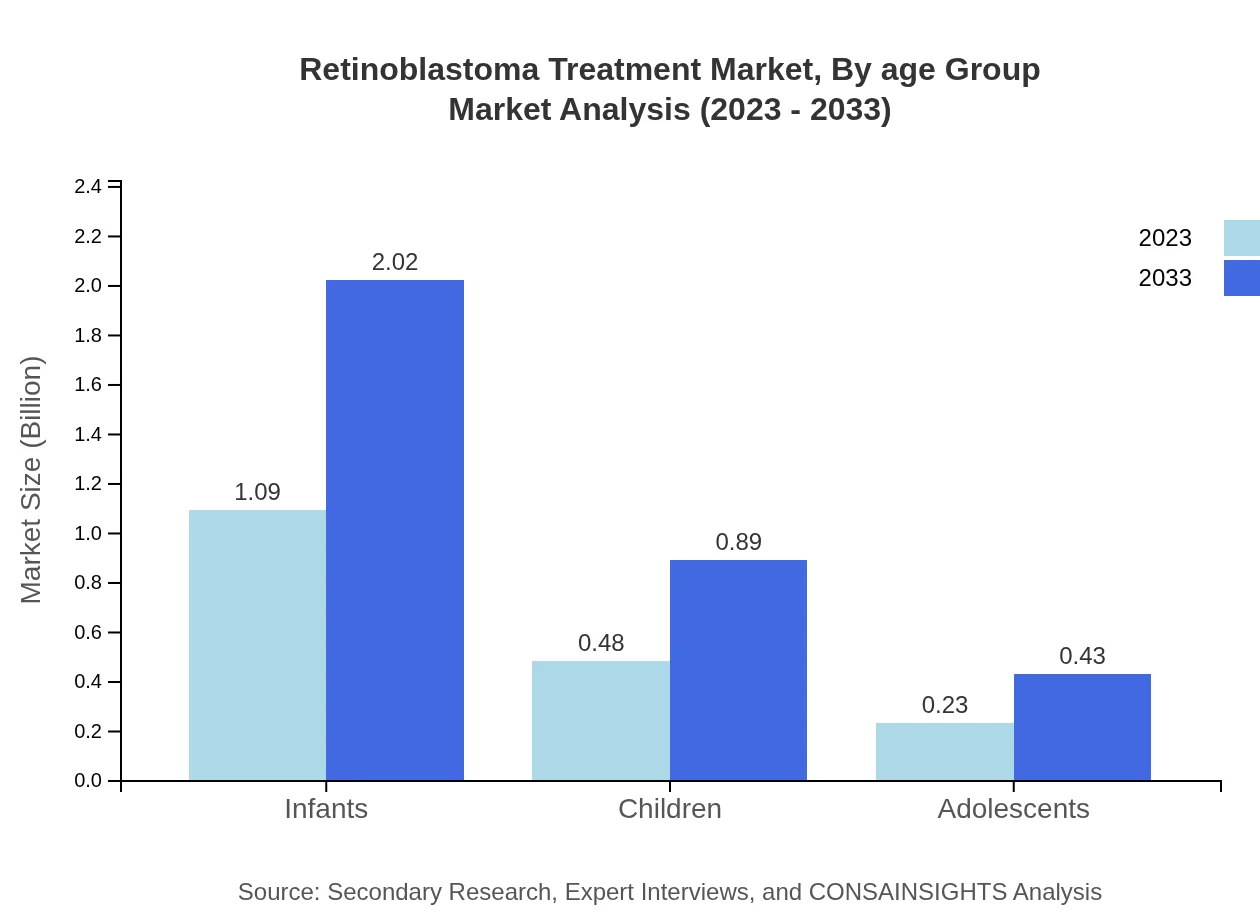
Retinoblastoma Treatment Market Analysis By Age Group
For age groups, infants represent the highest market share with USD 1.09 billion in 2023, reflecting 60.45% of the market and expected to double to USD 2.02 billion by 2033. The treatment for children comes next at USD 0.48 billion (26.69% share) and adolescents at USD 0.23 billion (12.86% share), with both groups projected for growth.
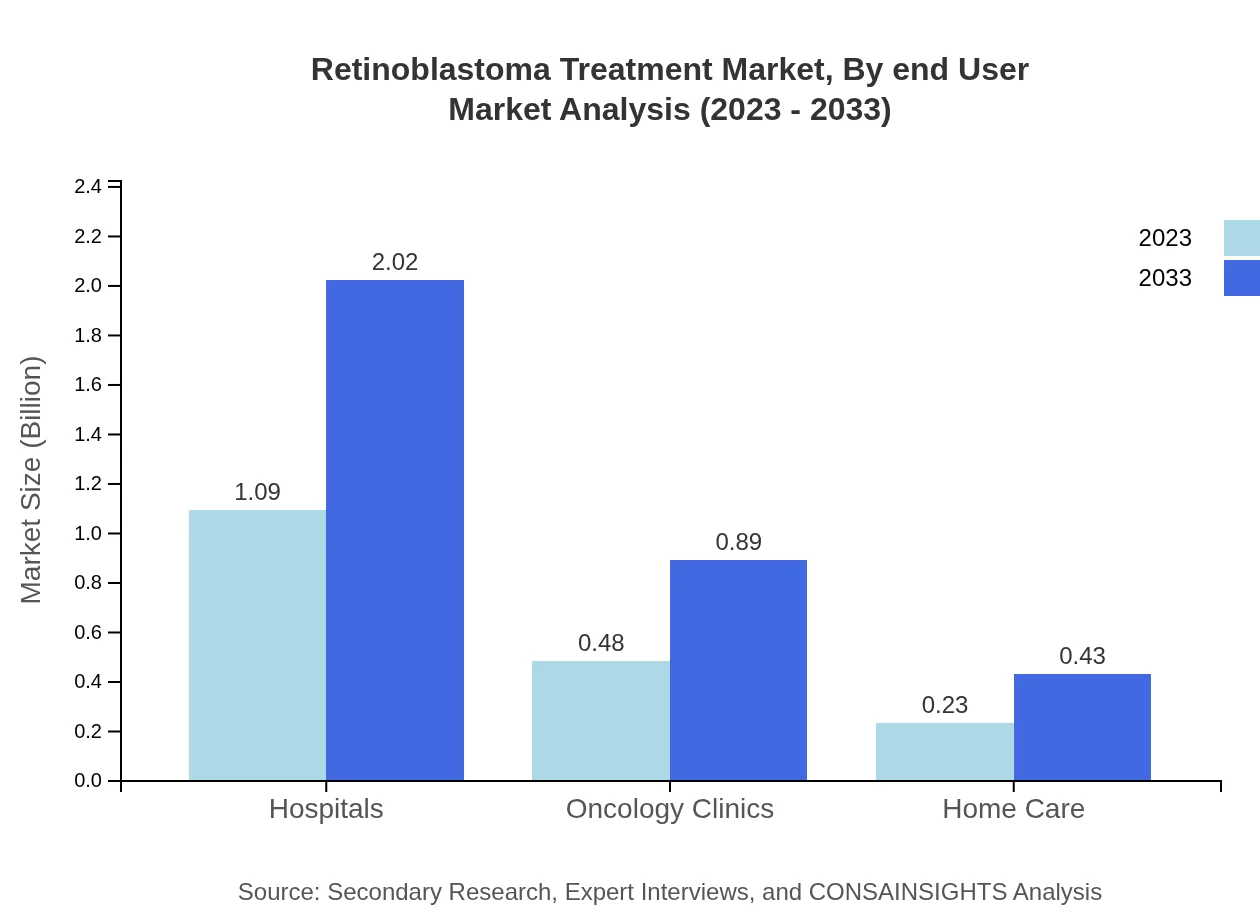
Retinoblastoma Treatment Market Analysis By End User
Hospitals dominate the end-user segment, contributing USD 1.09 billion in 2023, approximately 60.45% of the market, forecasted to reach USD 2.02 billion by 2033. Oncology clinics hold 26.69% of the market at USD 0.48 billion, expected to grow significantly in the next decade, while home care remains at 12.86% share.
Retinoblastoma Treatment Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Retinoblastoma Treatment Industry
Roche:
Roche is a pioneer in oncology, focusing on innovative therapies for pediatric cancer, including retinoblastoma. They contribute significantly through research and collaborations.Bristol-Myers Squibb:
Bristol-Myers Squibb specializes in cancer treatment therapies, with several ongoing projects aimed at improving outcomes in pediatric oncology.Novartis:
Novartis is actively involved in advancing retinoblastoma treatments through groundbreaking clinical trials and developing next-generation therapies.Genentech:
Genentech emphasizes genetic research and innovation in treatments, focusing on targeted therapies for improving retinoblastoma patient care.We're grateful to work with incredible clients.









FAQs
What is the market size of retinoblastoma treatment?
The global retinoblastoma treatment market is valued at approximately $1.8 billion in 2023, with a projected CAGR of 6.2% up to 2033. This growth is attributed to advancements in treatment methodologies and increased healthcare expenditures.
What are the key market players or companies in the retinoblastoma treatment industry?
Key players in the retinoblastoma treatment market include major pharmaceutical companies and biotechnology firms specializing in oncology treatments, which focus on developing innovative therapies and improving existing treatments.
What are the primary factors driving the growth in the retinoblastoma treatment industry?
Growth in the retinoblastoma treatment industry is driven by rising incidence rates, technological advancements in treatment options, increased healthcare spending, and heightened awareness regarding early detection and treatment of pediatric cancers.
Which region is the fastest Growing in the retinoblastoma treatment market?
The Asia Pacific region is experiencing significant growth, projected to increase from $0.30 billion in 2023 to $0.56 billion by 2033, as demand for improved healthcare and cancer treatment facilities expands.
Does ConsaInsights provide customized market report data for the retinoblastoma treatment industry?
Yes, ConsaInsights offers tailored market report data to cater to specific client needs in the retinoblastoma treatment industry, ensuring comprehensive insights based on unique business requirements and market conditions.
What deliverables can I expect from the retinoblastoma treatment market research project?
Given a comprehensive market research project, clients can expect detailed reports, market size analyses, growth forecasts, competitive landscape assessments, and customized insights tailored to their strategic needs.
What are the market trends of retinoblastoma treatment?
Current trends in the retinoblastoma treatment market include a shift towards more targeted therapies, increased focus on personalized medicine, and growing partnerships between biotechnology firms and research institutions to innovate treatment methodologies.