Rna Based Therapeutics Market Report
Published Date: 31 January 2026 | Report Code: rna-based-therapeutics
Rna Based Therapeutics Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the RNA-based therapeutics market, including insights on market size, growth trends, and forecasts from 2023 to 2033. It covers industry analysis, segmentation, regional insights, and key market leaders in the RNA therapeutic space.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
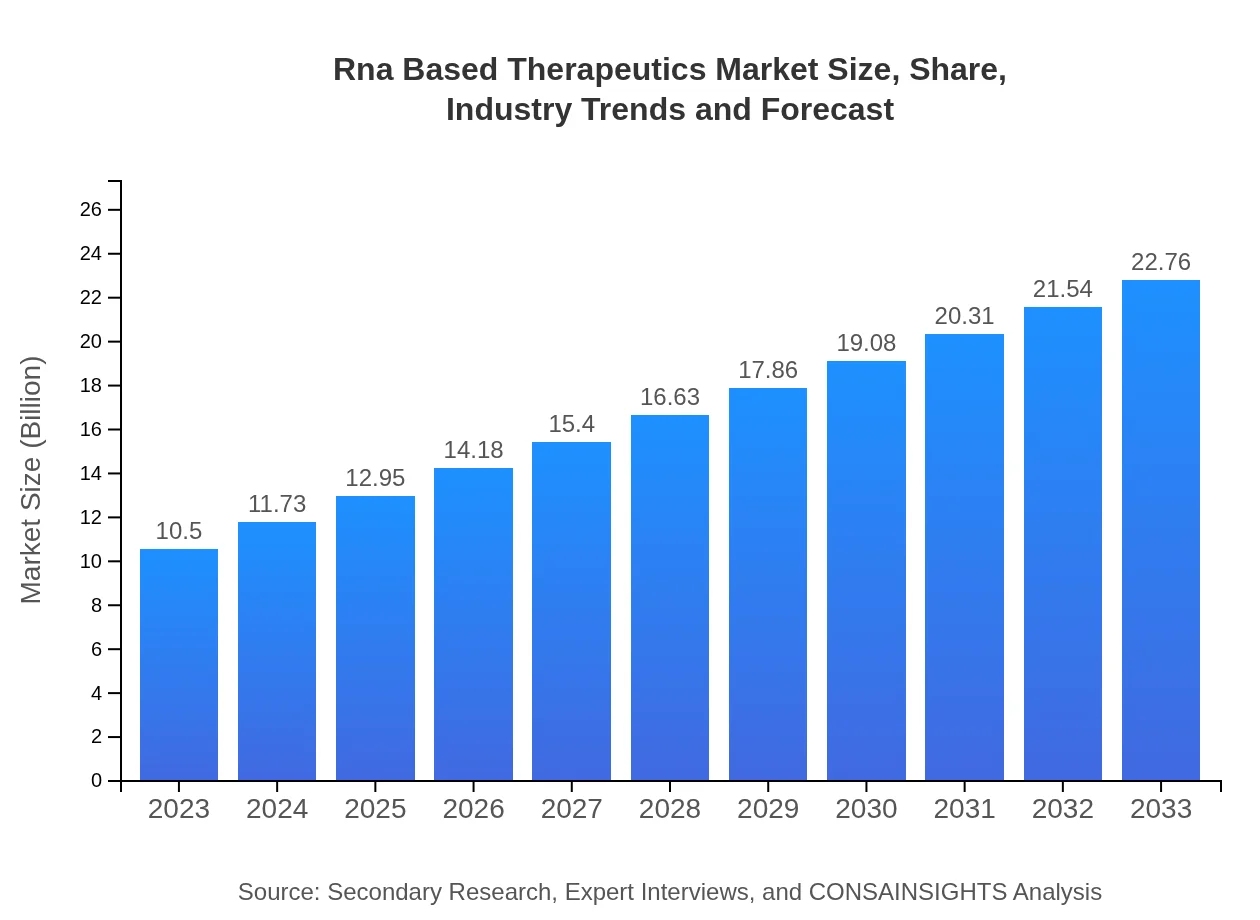
| 2023 Market Size | $10.50 Billion |
| CAGR (2023-2033) | 7.8% |
| 2033 Market Size | $22.76 Billion |
| Top Companies | Moderna, Inc., Alnylam Pharmaceuticals, BioNTech SE, Sangamo Therapeutics, Ionis Pharmaceuticals |
| Last Modified Date | 31 January 2026 |
RNA Based Therapeutics Market Overview
Customize Rna Based Therapeutics Market Report market research report
- ✔ Get in-depth analysis of Rna Based Therapeutics market size, growth, and forecasts.
- ✔ Understand Rna Based Therapeutics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Rna Based Therapeutics
What is the Market Size & CAGR of RNA Based Therapeutics market in 2023?
RNA Based Therapeutics Industry Analysis
RNA Based Therapeutics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
RNA Based Therapeutics Market Analysis Report by Region
Europe Rna Based Therapeutics Market Report:
The European market is estimated at $2.72 billion in 2023 and expected to grow to $5.90 billion by 2033, driven by strong healthcare policies supporting biotechnology innovation and a collaborative environment between public and private entities.Asia Pacific Rna Based Therapeutics Market Report:
In the Asia Pacific region, the RNA-based therapeutics market is valued at approximately $2.04 billion in 2023 and is expected to reach $4.43 billion by 2033. The region is characterized by increasing investments in biotechnology, rising incidences of chronic diseases, and government initiatives aimed at enhancing healthcare infrastructure.North America Rna Based Therapeutics Market Report:
North America holds a dominant position in the RNA therapeutics market, with an estimated market value of $3.90 billion in 2023, projected to grow to approximately $8.46 billion by 2033. The strong presence of key pharmaceutical firms, along with significant funding for RNA research, positions North America as a leader in innovation.South America Rna Based Therapeutics Market Report:
The South American RNA-based therapeutics market is relatively smaller, estimated at $0.44 billion in 2023, with projections reaching $0.96 billion by 2033. The growth potential in this region is bolstered by growing healthcare access, although regulatory challenges may impede swift advancements.Middle East & Africa Rna Based Therapeutics Market Report:
The Middle East and Africa region is expected to see market growth from $1.39 billion in 2023 to around $3.01 billion by 2033. Efforts to enhance regional healthcare infrastructure and increasing cooperation with international firms will likely facilitate market expansion.Tell us your focus area and get a customized research report.
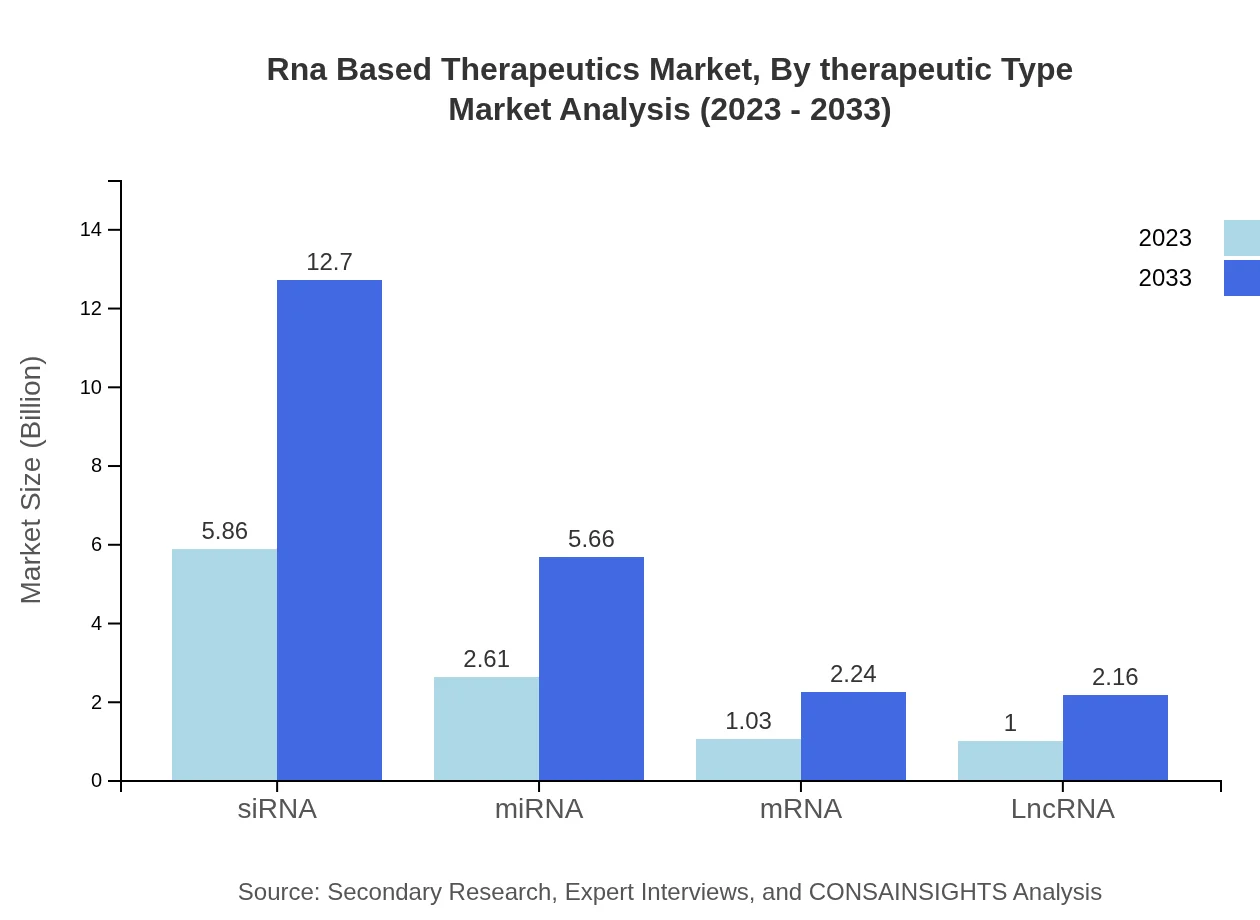
Rna Based Therapeutics Market Analysis By Therapeutic Type
The RNA-based therapeutics market is primarily driven by siRNA, mRNA, and miRNA segments. siRNA dominates the market, accounting for 55.78% of the therapeutic type share in 2023. mRNA, being a novel platform with applications in vaccines and therapies, is also rapidly gaining traction. miRNA, although smaller in share, offers substantial prospects for targeted therapies in various diseases.
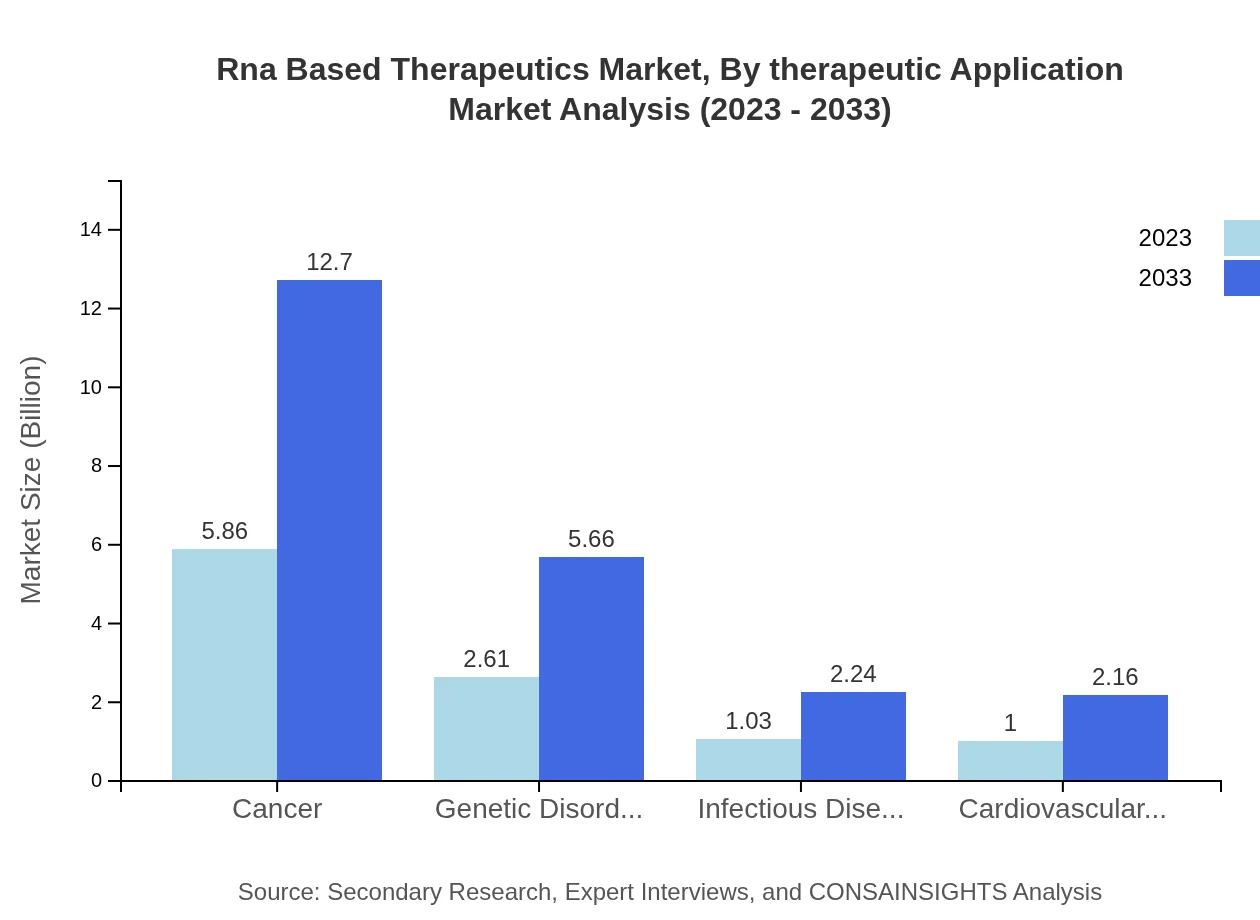
Rna Based Therapeutics Market Analysis By Therapeutic Application
Cancer remains the leading application for RNA-based therapeutics, holding a substantial market share of 55.78% in 2023. The rise in RNA therapeutics for genetic disorders and infectious diseases also contributes significantly, reflecting the versatility of RNA technologies in addressing diverse medical challenges.
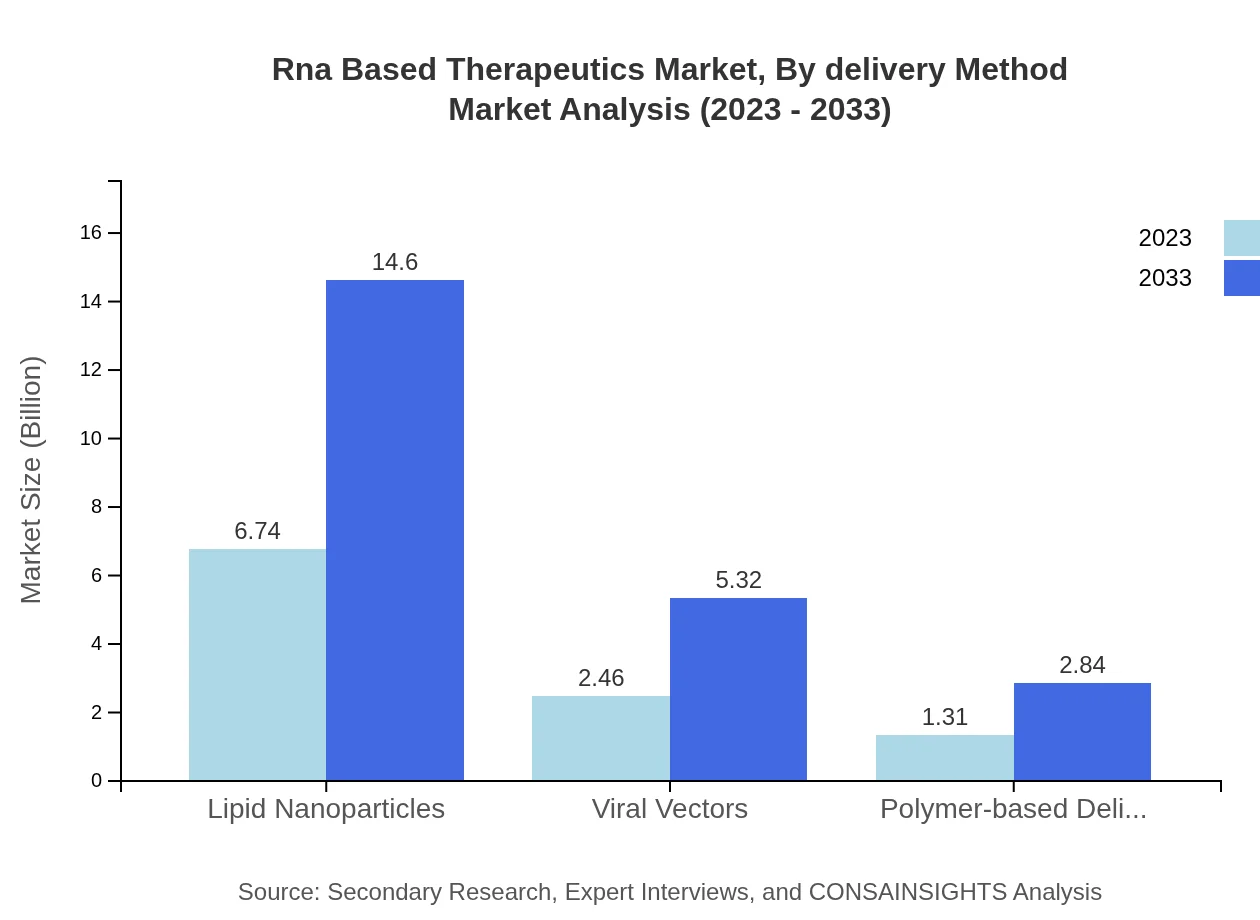
Rna Based Therapeutics Market Analysis By Delivery Method
Lipid nanoparticles are the leading delivery method for RNA-based therapeutics, as they enhance stability and cellular uptake of RNA molecules. Other methods include viral vectors and polymer-based delivery systems, each playing a crucial role in the efficacy of RNA therapeutics.
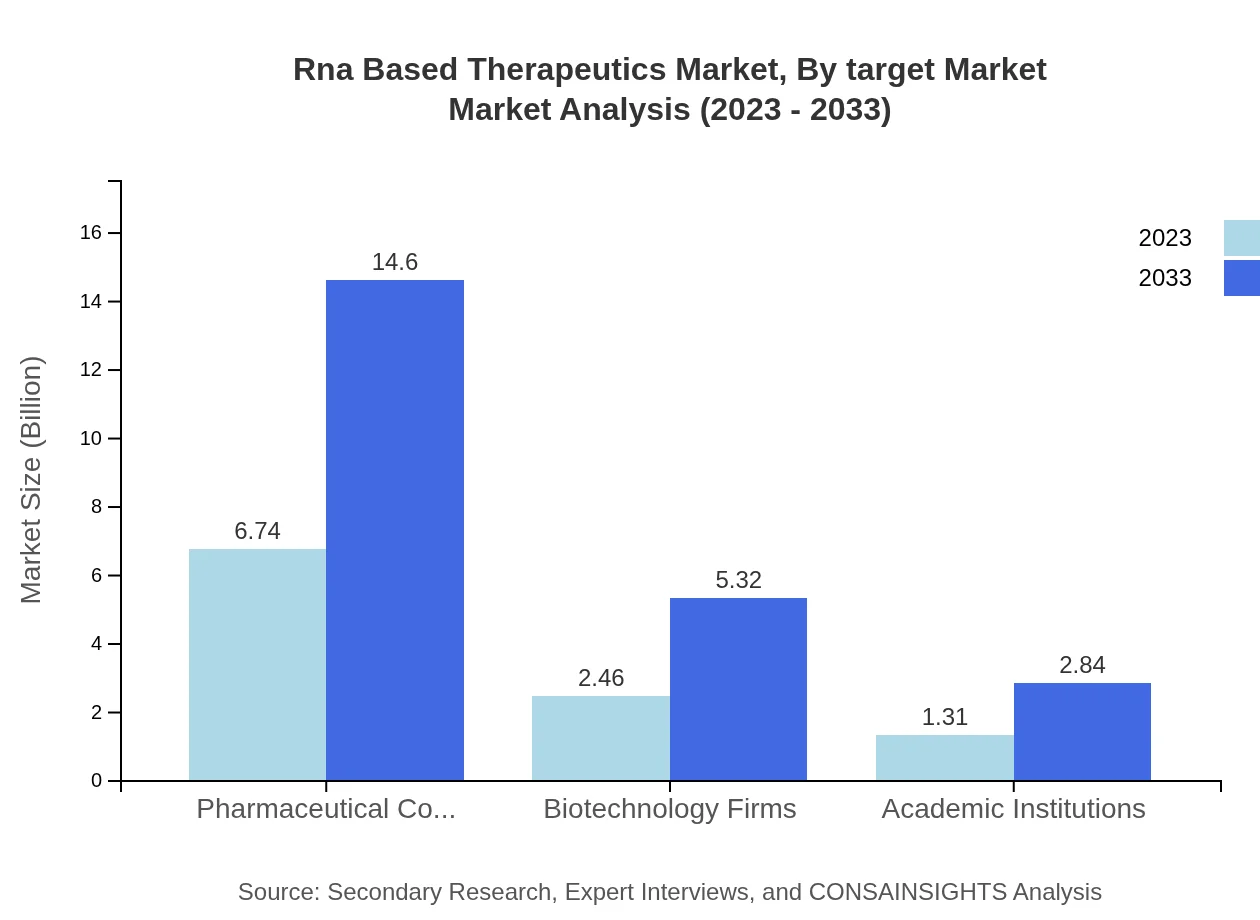
Rna Based Therapeutics Market Analysis By Target Market
The major target markets for RNA-based therapeutics encompass oncology, genetic disorders, and infectious diseases. The substantial focus on oncology reflects the high unmet medical needs in cancer therapies, while genetic disorders are increasingly being targeted due to the potential for corrective therapies.
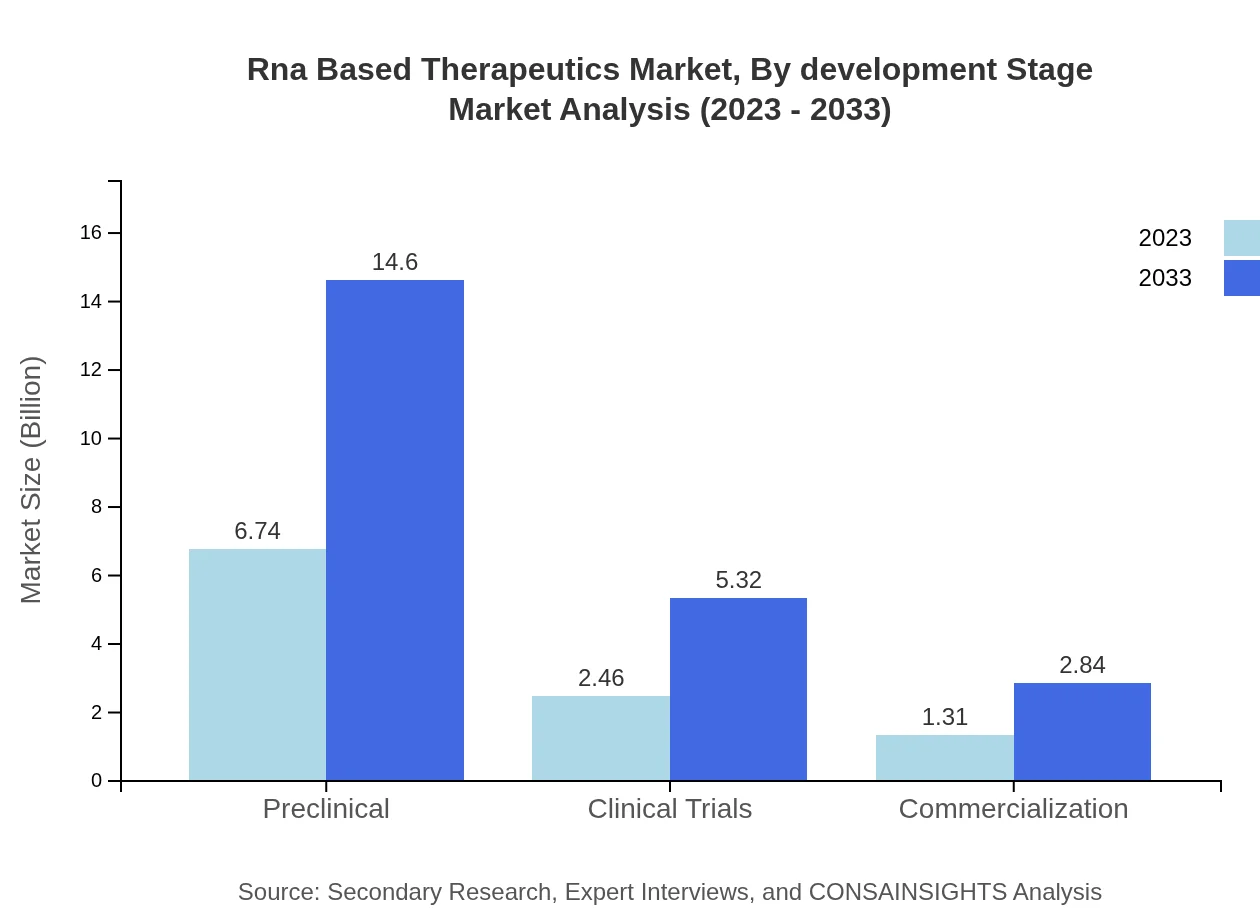
Rna Based Therapeutics Market Analysis By Development Stage
The RNA-based therapeutics market is segmented by development stage into preclinical, clinical trials, and commercialization. Preclinical phases dominate the market with a share of 64.15%, followed by ongoing clinical trials which provide insights into the effectiveness of RNA therapeutics.
RNA Based Therapeutics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in RNA Based Therapeutics Industry
Moderna, Inc.:
Moderna is a pioneering biotechnology company focusing on the development of mRNA-based vaccines and therapeutics. The company gained prominence with its COVID-19 vaccine.Alnylam Pharmaceuticals:
Alnylam is a leader in RNA interference therapies and is known for its transformative medicines aimed at treating rare genetic diseases.BioNTech SE:
BioNTech specializes in mRNA-based pharmaceuticals, particularly in oncology and infectious diseases, leading innovations in personalized medicine.Sangamo Therapeutics:
Sangamo focuses on genomic therapies and is developing innovative RNA-based solutions for genetic disorders.Ionis Pharmaceuticals:
Ionis is a pioneer in the development of antisense therapeutics and RNA-targeted drugs to treat life-threatening diseases.We're grateful to work with incredible clients.









FAQs
What is the market size of RNA Based therapeutics?
The RNA-based therapeutics market is valued at approximately $10.5 billion in 2023 and is projected to grow at a CAGR of 7.8%, reaching around $23.2 billion by 2033.
What are the key market players or companies in the RNA Based therapeutics industry?
Key players in the RNA-based therapeutics industry include major pharmaceutical companies and biotechnology firms. Notable companies focus on various delivery systems like Lipid Nanoparticles and Viral Vectors, enhancing therapeutic efficiency and efficacy.
What are the primary factors driving the growth in the RNA Based therapeutics industry?
The growth in the RNA-based therapeutics market is driven by increasing investments in R&D, rising prevalence of genetic disorders and cancers, advancements in delivery technologies, and the growing popularity of personalized medicine.
Which region is the fastest Growing in the RNA Based therapeutics?
The fastest-growing region in the RNA-based therapeutics market is North America, with a market size of $3.90 billion in 2023 projected to grow to $8.46 billion by 2033. Europe and the Asia-Pacific regions also show significant growth potential.
Does ConsaInsights provide customized market report data for the RNA Based therapeutics industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the RNA-based therapeutics industry, enabling stakeholders to access relevant insights for strategic decision-making.
What deliverables can I expect from this RNA Based therapeutics market research project?
Deliverables from the RNA-based therapeutics market research project include a detailed market analysis, trend reports, competitive landscape insights, and recommendations for potential market entry strategies.
What are the market trends of RNA Based therapeutics?
Current market trends in RNA-based therapeutics highlight a shift towards innovative delivery methods, increased focus on mRNA technologies, and the growth of personalized treatment approaches catering to specific patient needs.