Rubella Diagnostic Testing Market Report
Published Date: 31 January 2026 | Report Code: rubella-diagnostic-testing
Rubella Diagnostic Testing Market Size, Share, Industry Trends and Forecast to 2033
This report delves into the Rubella Diagnostic Testing market, providing comprehensive insights, analysis on market trends, growth forecasts, and detailed evaluation from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
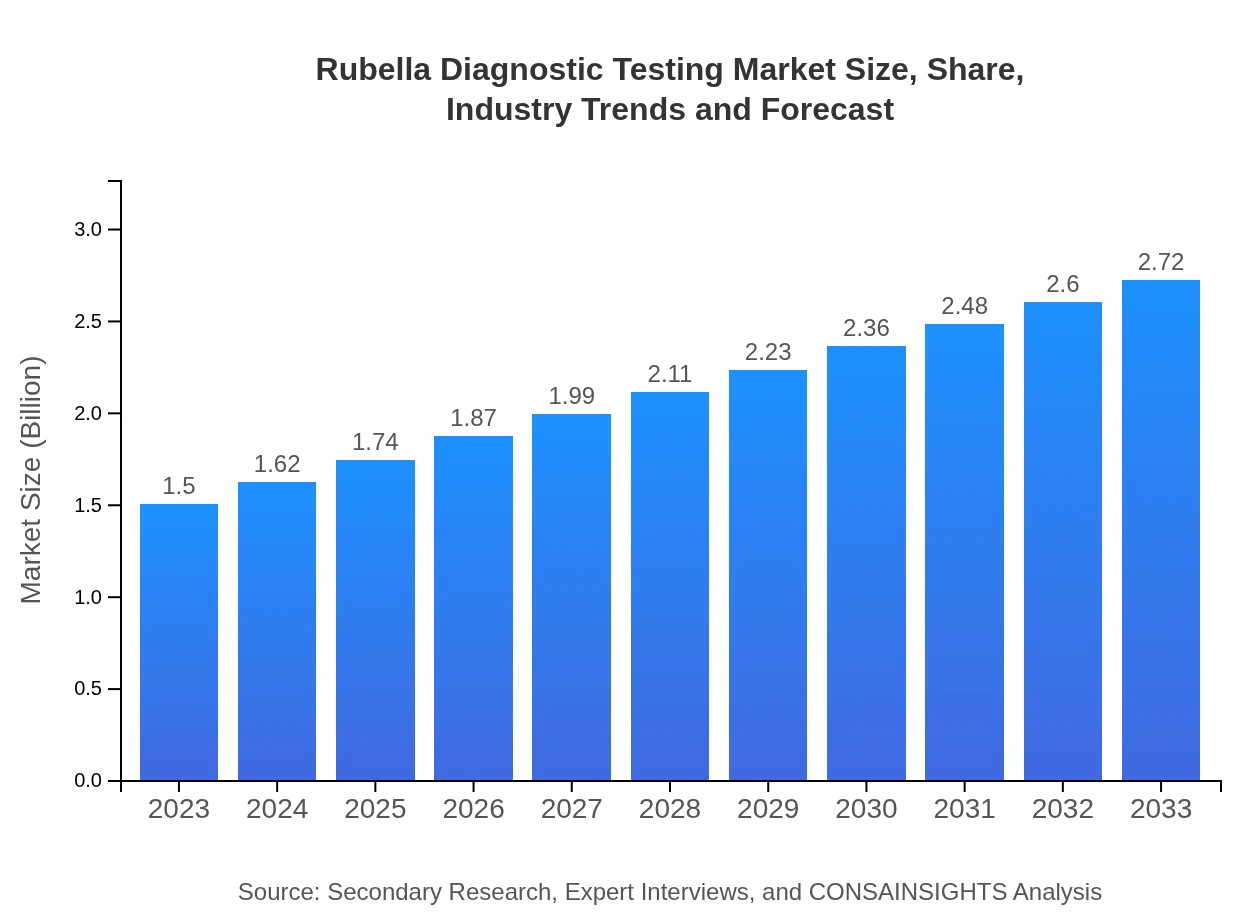
| 2023 Market Size | $1.50 Billion |
| CAGR (2023-2033) | 6.0% |
| 2033 Market Size | $2.72 Billion |
| Top Companies | Roche Diagnostics, Abbott Laboratories, Siemens Healthineers, Thermo Fisher Scientific |
| Last Modified Date | 31 January 2026 |
Rubella Diagnostic Testing Market Overview
Customize Rubella Diagnostic Testing Market Report market research report
- ✔ Get in-depth analysis of Rubella Diagnostic Testing market size, growth, and forecasts.
- ✔ Understand Rubella Diagnostic Testing's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Rubella Diagnostic Testing
What is the Market Size & CAGR of Rubella Diagnostic Testing market in 2023?
Rubella Diagnostic Testing Industry Analysis
Rubella Diagnostic Testing Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Rubella Diagnostic Testing Market Analysis Report by Region
Europe Rubella Diagnostic Testing Market Report:
In Europe, the market size is expected to grow from USD 0.51 billion in 2023 to USD 0.93 billion by 2033, fostering a strong culture of public health initiatives and vaccination campaigns that support rubella testing.Asia Pacific Rubella Diagnostic Testing Market Report:
In the Asia Pacific region, the Rubella Diagnostic Testing market was valued at approximately USD 0.28 billion in 2023 and is expected to reach USD 0.51 billion by 2033, driven by increasing awareness and healthcare infrastructure improvements.North America Rubella Diagnostic Testing Market Report:
The North American market is projected to grow from USD 0.53 billion in 2023 to USD 0.96 billion by 2033, with a robust healthcare framework and strong research capabilities enhancing diagnostic efforts.South America Rubella Diagnostic Testing Market Report:
The South American market, albeit facing challenges with low testing rates, shows potential for growth from USD 0.00 billion in 2023, with expectations of consistent development by 2033 as vaccination programs expand.Middle East & Africa Rubella Diagnostic Testing Market Report:
The Middle East and Africa markets are expected to see growth from USD 0.18 billion in 2023 to USD 0.33 billion by 2033, benefited by international health organizations' support in raising awareness about rubella control.Tell us your focus area and get a customized research report.
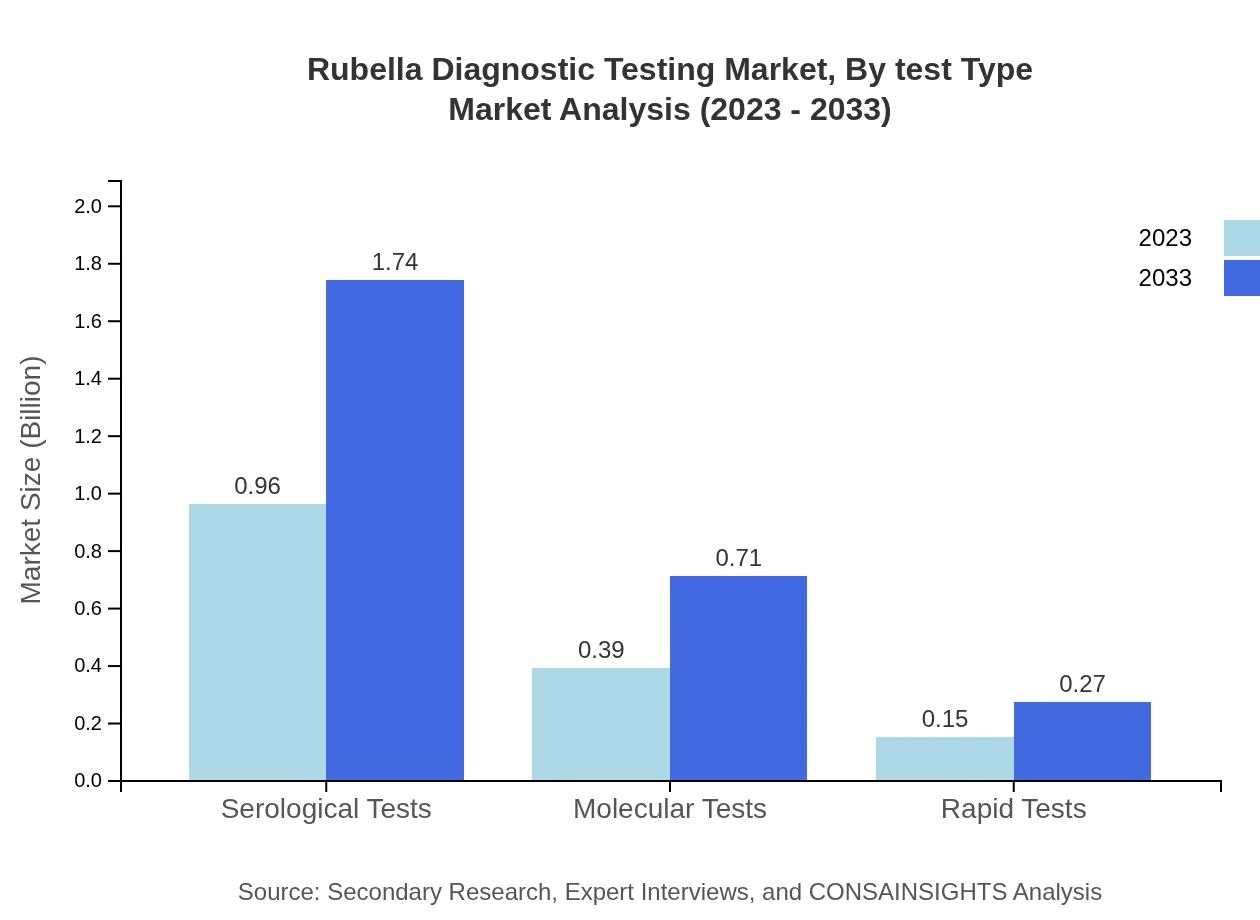
Rubella Diagnostic Testing Market Analysis By Test Type
The by-test-type analysis reveals that serological tests dominate the market with a size of USD 0.96 billion in 2023, expected to grow to USD 1.74 billion by 2033, holding a market share of 63.87%. Traditional lab methods also have a significant share at 85.72% in 2023, expected to rise to 85.72% by 2033, indicating their ongoing relevance. Meanwhile, molecular tests, though smaller ($0.39 billion in 2023), will increase to USD 0.71 billion by 2033, reflecting an emerging trend towards advanced diagnostic methods.
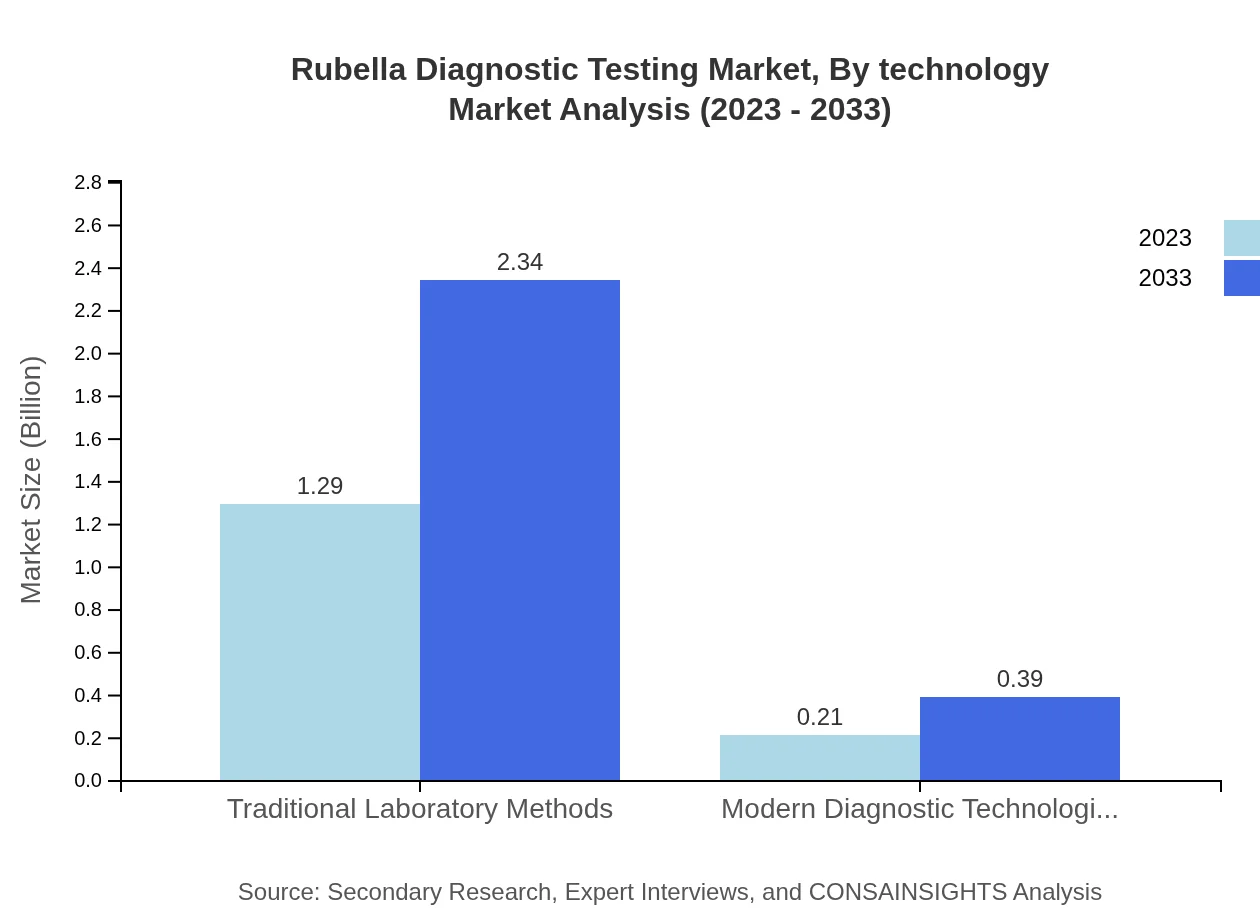
Rubella Diagnostic Testing Market Analysis By Technology
Analysing by technology, traditional laboratory methods lead with a revenue of USD 1.29 billion in 2023, projected to grow to USD 2.34 billion by 2033, capturing 85.72% of the market. Next are molecular tests, which are gaining ground with a projected market size of USD 0.71 billion by 2033. Rapid tests, considered essential for rapid diagnosis, represent 9.9% of the market and are predicted to reach USD 0.27 billion.
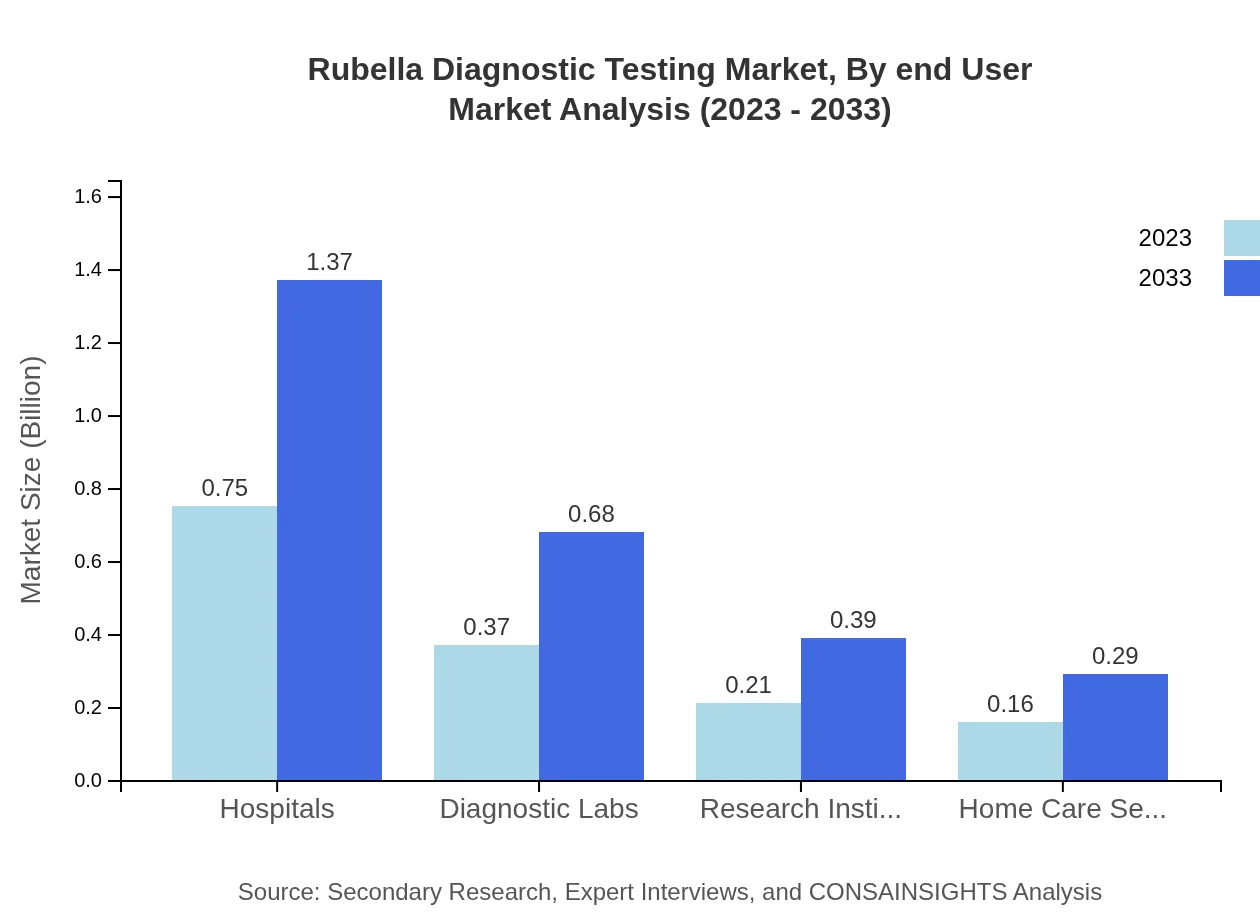
Rubella Diagnostic Testing Market Analysis By End User
Segmenting by end-users, hospitals represent the largest segment with a size of USD 0.75 billion in 2023, expected to increase to USD 1.37 billion by 2033, maintaining 50.19% market share. Diagnostic labs also play an important role, boasting a market size of USD 0.37 billion expected to reach $0.68 billion by 2033, while research institutes follow with USD 0.21 billion with growth expected to USD 0.39 billion.
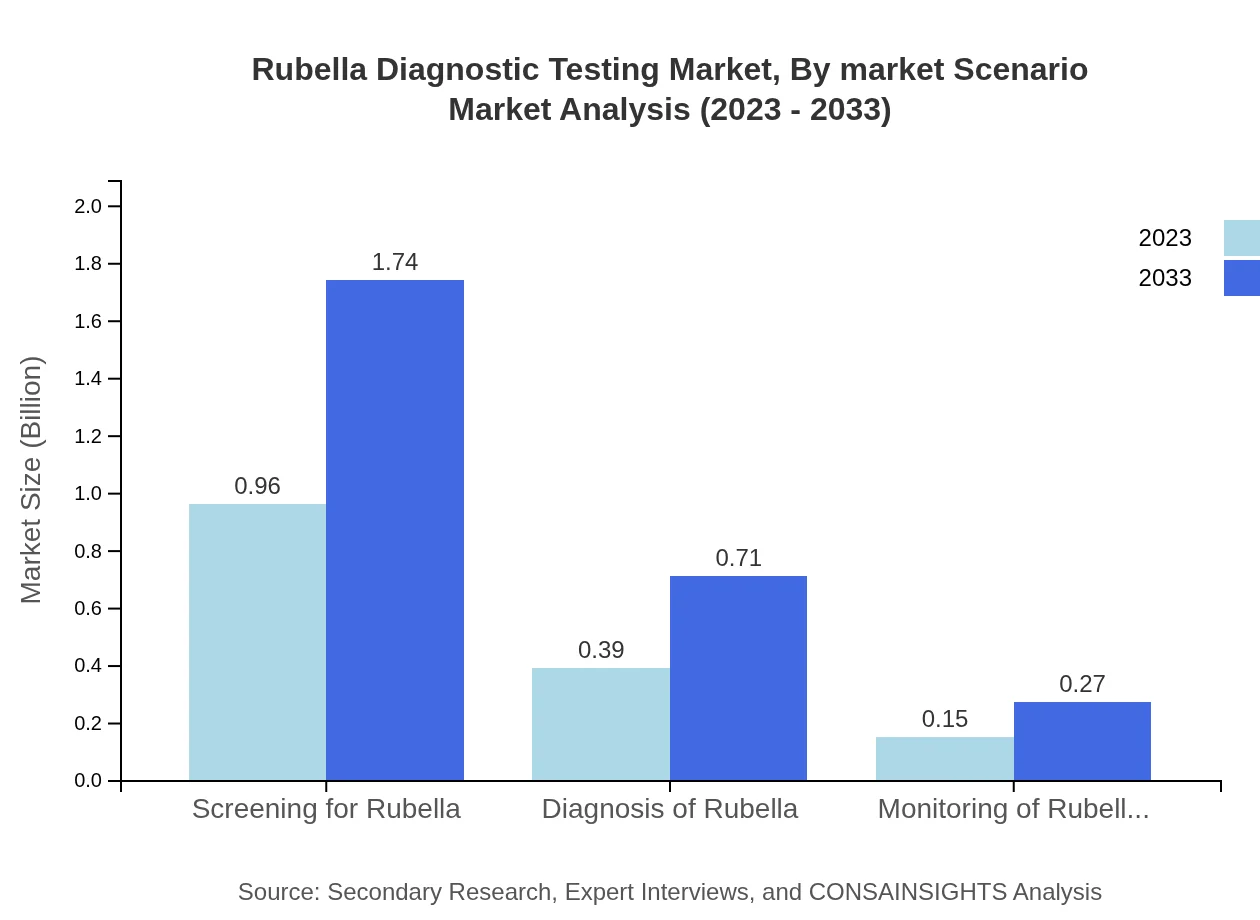
Rubella Diagnostic Testing Market Analysis By Market Scenario
Market scenarios highlight that screening for rubella dominates with a size of USD 0.96 billion in 2023, predicted to reach USD 1.74 billion by 2033. This segment underscores the importance of early detection and control measures against rubella. Additional insights into the diagnosis and monitoring segments indicate potential growth but at a smaller scale, reflecting healthcare systems' evolving capabilities in managing rubella effectively.
Rubella Diagnostic Testing Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Rubella Diagnostic Testing Industry
Roche Diagnostics:
A veteran in the diagnostics field, Roche has a comprehensive range of rubella diagnostics solutions, focusing on innovative serological tests that improve outcome reliability.Abbott Laboratories:
Abbott's contributions include advanced molecular diagnostics, offering cutting-edge technologies that enhance rubella testing accuracy and speed, promoting public health interventions.Siemens Healthineers:
Siemens provides a variety of laboratory solutions, characterized by high-throughput testing that supports effective rubella screening and diagnosis in various healthcare settings.Thermo Fisher Scientific:
Known for its innovative products, Thermo Fisher has developed various serological and molecular diagnostic solutions that cater to the growing demands of rubella testing.We're grateful to work with incredible clients.









FAQs
What is the market size of rubella Diagnostic Testing?
The rubella diagnostic testing market is valued at approximately $1.5 billion in 2023, with a projected CAGR of 6.0%, indicating steadily increasing demand over the next several years.
What are the key market players or companies in this rubella Diagnostic Testing industry?
Key players in the rubella diagnostic testing market include established medical device manufacturers, diagnostic laboratories, and market entry startups focused on innovative testing technologies and services.
What are the primary factors driving the growth in the rubella Diagnostic Testing industry?
Growth is driven by increased awareness of rubella, rising vaccination programs, advancements in diagnostic technologies, and government initiatives aimed at controlling infectious diseases.
Which region is the fastest Growing in the rubella Diagnostic Testing?
The fastest-growing region in the rubella diagnostic testing market is North America, projected to grow from $0.53 billion in 2023 to $0.96 billion by 2033, driven by supporting healthcare infrastructure.
Does ConsaInsights provide customized market report data for the rubella Diagnostic Testing industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the rubella diagnostic testing industry to help stakeholders make informed decisions.
What deliverables can I expect from this rubella Diagnostic Testing market research project?
Deliverables include comprehensive market analysis, segment-specific data, trend insights, competitor analysis, and actionable recommendations tailored for stakeholders in the rubella diagnostic testing space.
What are the market trends of rubella Diagnostic Testing?
Current trends include the rising adoption of molecular and serological tests, increasing reliance on rapid testing methods, and the integration of modern diagnostic technologies for enhanced accuracy.