Rubella Vaccine Human Diploid Cell Market Report
Published Date: 31 January 2026 | Report Code: rubella-vaccine-human-diploid-cell
Rubella Vaccine Human Diploid Cell Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Rubella Vaccine Human Diploid Cell market from 2023 to 2033. It highlights market trends, forecasts, and insights regarding market conditions, major players, and technological advancements within the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
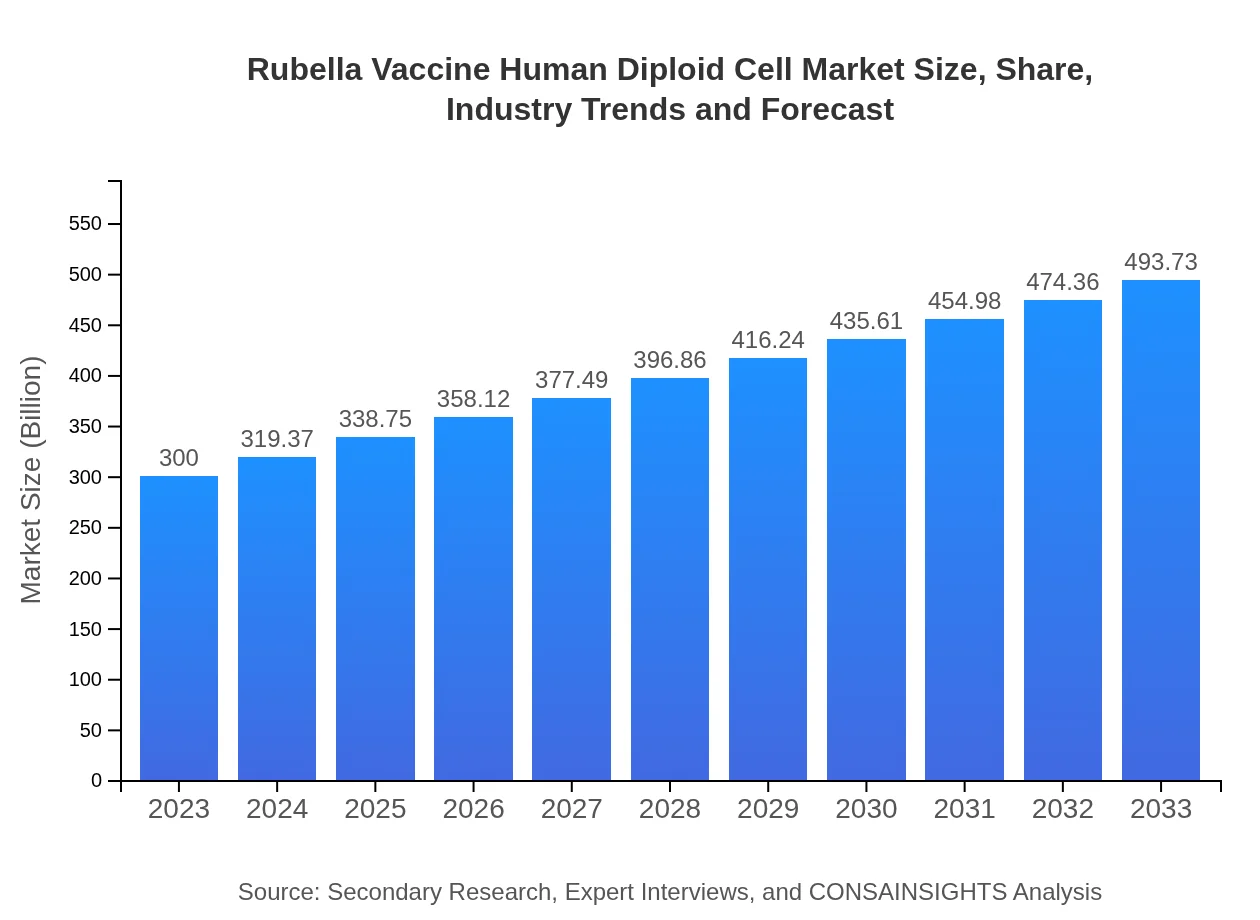
| 2023 Market Size | $300.00 Million |
| CAGR (2023-2033) | 5% |
| 2033 Market Size | $493.73 Million |
| Top Companies | Merck & Co., Inc., Sanofi Pasteur, GlaxoSmithKline (GSK), Pfizer Inc. |
| Last Modified Date | 31 January 2026 |
Rubella Vaccine Human Diploid Cell Market Overview
Customize Rubella Vaccine Human Diploid Cell Market Report market research report
- ✔ Get in-depth analysis of Rubella Vaccine Human Diploid Cell market size, growth, and forecasts.
- ✔ Understand Rubella Vaccine Human Diploid Cell's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Rubella Vaccine Human Diploid Cell
What is the Market Size & CAGR of Rubella Vaccine Human Diploid Cell market in 2023?
Rubella Vaccine Human Diploid Cell Industry Analysis
Rubella Vaccine Human Diploid Cell Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Rubella Vaccine Human Diploid Cell Market Analysis Report by Region
Europe Rubella Vaccine Human Diploid Cell Market Report:
The European market for Rubella Vaccine Human Diploid Cell was valued at 86.31 million in 2023 and is projected to reach 142.05 million by 2033. The region benefits from strong regulatory support and a comprehensive healthcare system focused on vaccination.Asia Pacific Rubella Vaccine Human Diploid Cell Market Report:
In the Asia-Pacific region, the market for the Rubella Vaccine Human Diploid Cell was valued at 59.64 million in 2023 and is expected to grow to 98.15 million by 2033. This growth is driven by increasing government initiatives to boost immunization rates and a rising awareness of infectious diseases.North America Rubella Vaccine Human Diploid Cell Market Report:
In North America, the market stood at 105.60 million in 2023 and is expected to rise to 173.79 million by 2033. Factors influencing this growth include advanced healthcare infrastructure, a high adoption rate of vaccinations, and ongoing public health campaigns.South America Rubella Vaccine Human Diploid Cell Market Report:
The South American market was valued at 14.04 million in 2023, with projections suggesting a lift to 23.11 million by 2033. The growth in this region is attributed to efforts aimed at enhancing vaccine accessibility and affordability in public health settings.Middle East & Africa Rubella Vaccine Human Diploid Cell Market Report:
The Middle East and Africa market was valued at 34.41 million in 2023, with forecasts indicating growth to 56.63 million by 2033. This growth highlights the commitment to improving health outcomes through vaccination programs in emerging economies.Tell us your focus area and get a customized research report.
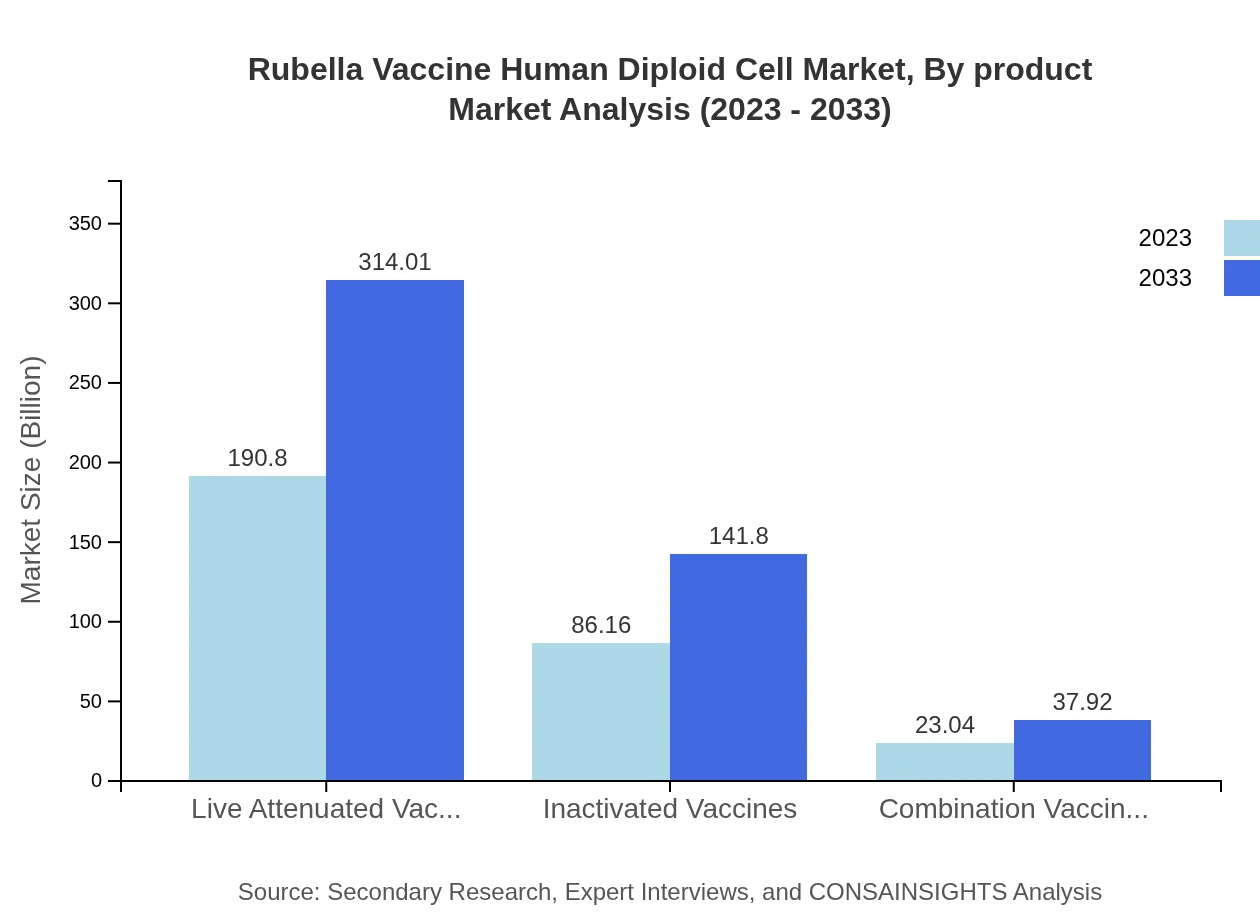
Rubella Vaccine Human Diploid Cell Market Analysis By Product
The Rubella Vaccine market is segmented into various product types including Live Attenuated Vaccines, Inactivated Vaccines, and Combination Vaccines. In 2023, live attenuated vaccines accounted for 190.80 million, while inactivated vaccines reached 86.16 million. By 2033, live attenuated vaccines are expected to grow to 314.01 million, showcasing their predominance in the market.
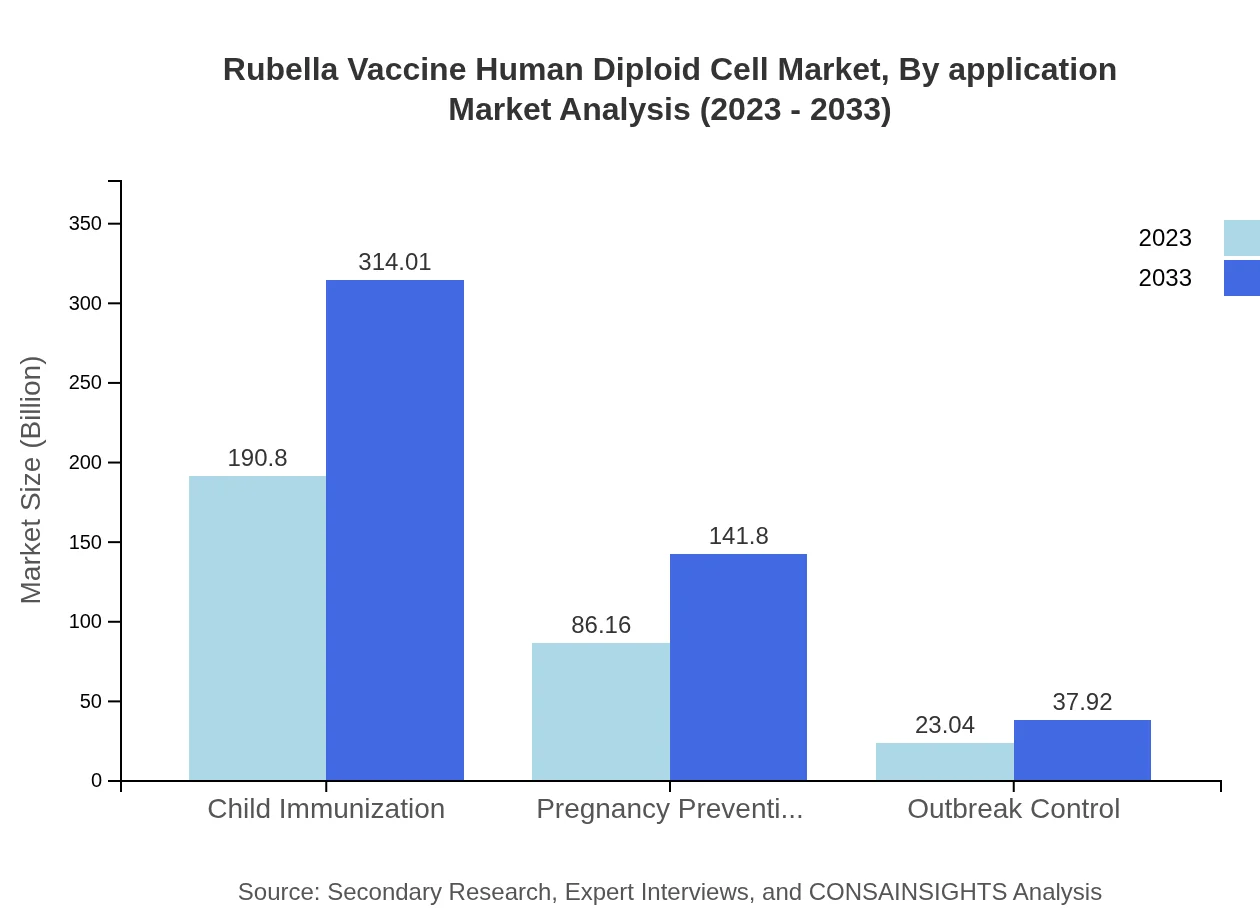
Rubella Vaccine Human Diploid Cell Market Analysis By Application
By application, the market is examined across Child Immunization, Pregnancy Prevention and Management, and Outbreak Control. Child Immunization leads in size with 190.80 million in 2023, anticipated to grow to 314.01 million by 2033, indicating a strong focus on childhood vaccines.
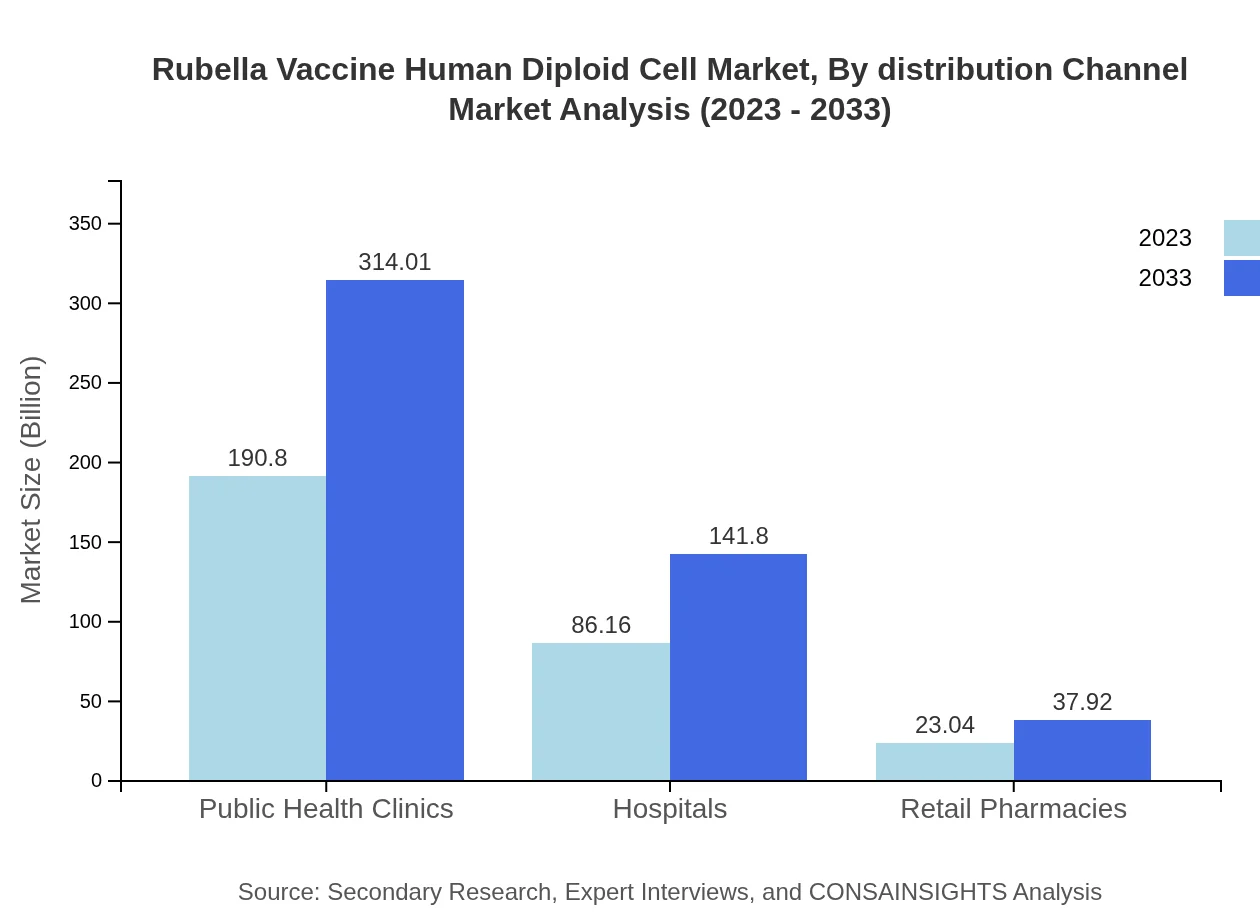
Rubella Vaccine Human Diploid Cell Market Analysis By Distribution Channel
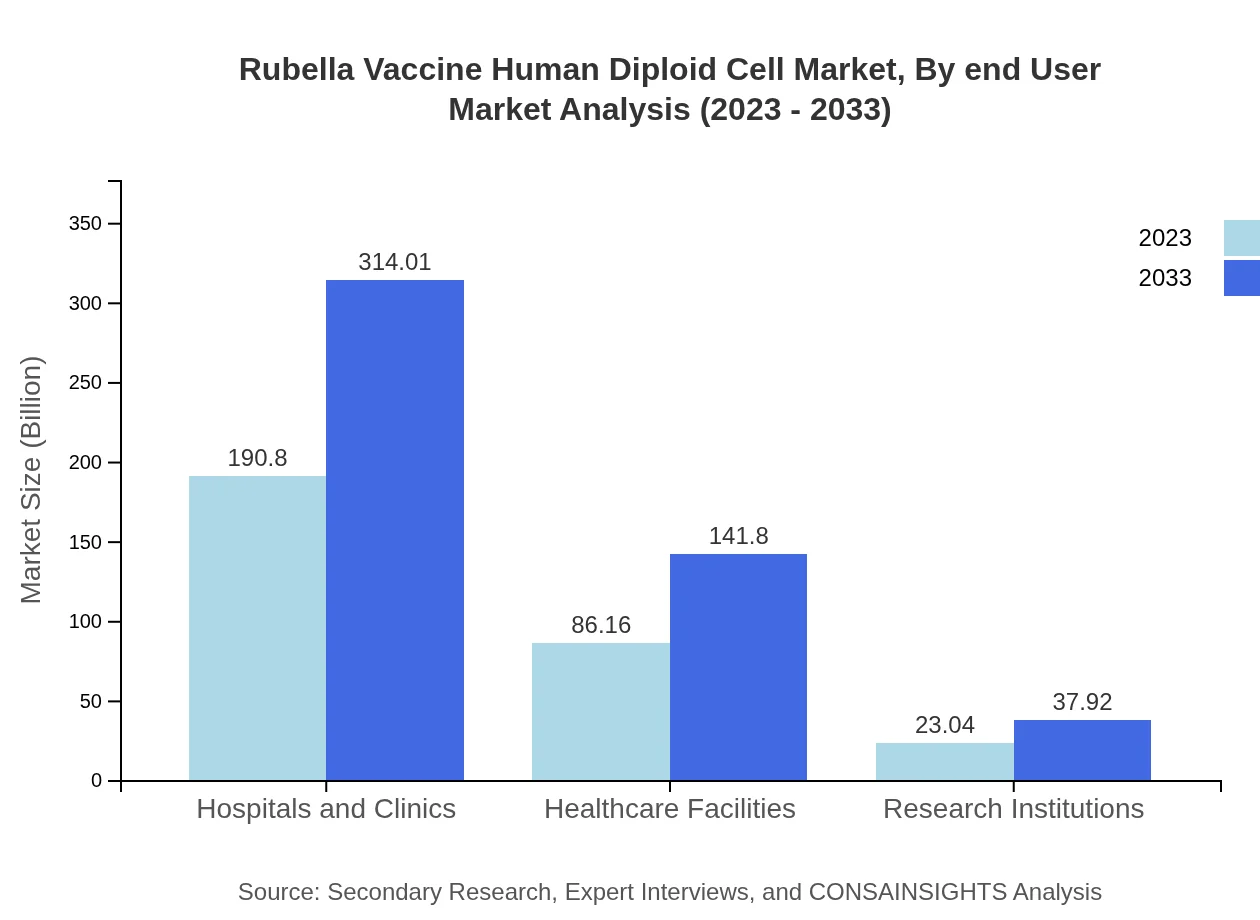
The distribution channels for vaccines encompass Hospitals and Clinics, Healthcare Facilities, Research Institutions, and Public Health Clinics. The hospital segment shows substantial engagement with 190.80 million in 2023, expected to reach 314.01 million by 2033.
Rubella Vaccine Human Diploid Cell Market Analysis By End User
End-users include hospitals, healthcare systems, and public health organizations. Hospitals, holding a significant share with 86.16 million in 2023, exhibit a strong forecast due to their role in administering vaccines directly to patients.
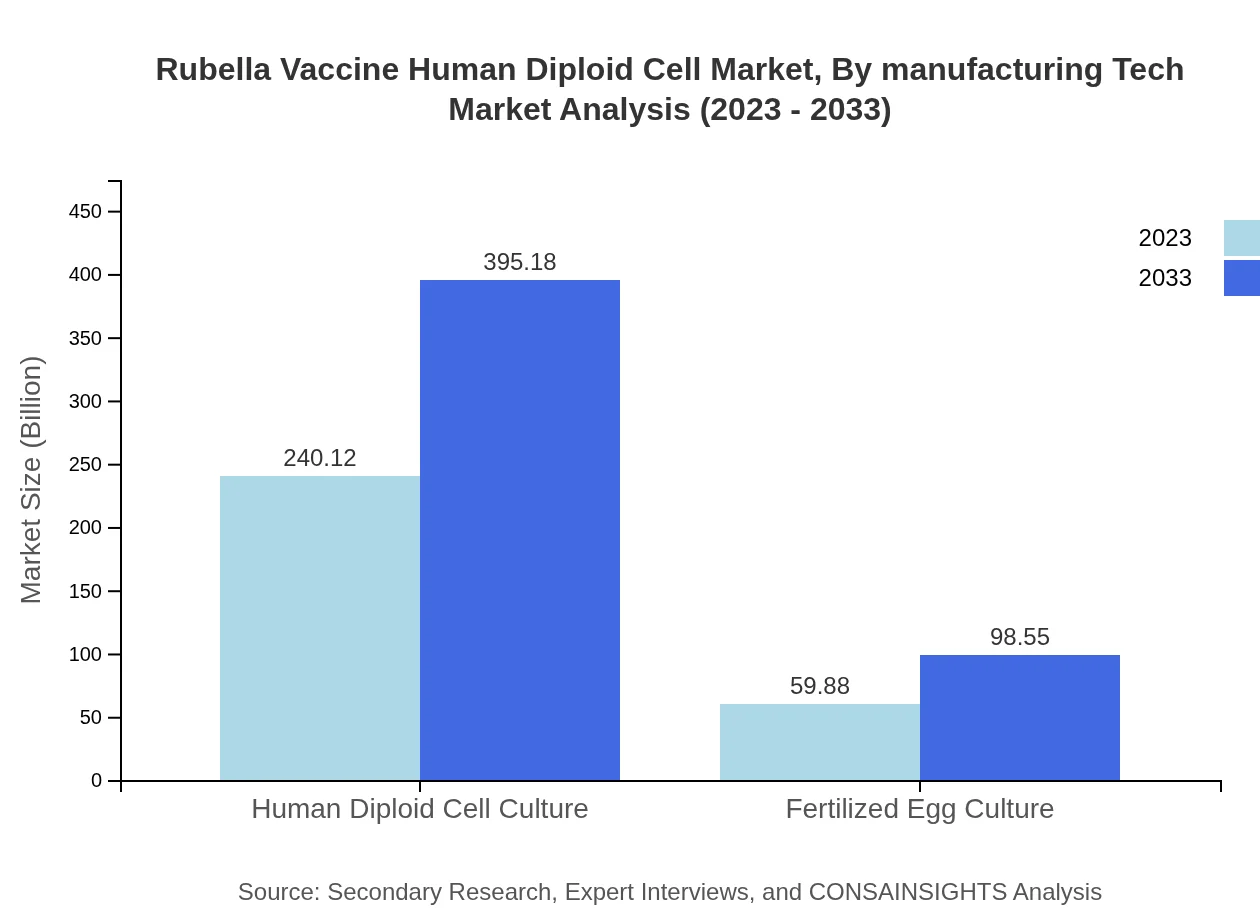
Rubella Vaccine Human Diploid Cell Market Analysis By Manufacturing Tech
The manufacturing technology segment covers Human Diploid Cell Culture and Fertilized Egg Culture. The human diploid cell market was valued at 240.12 million in 2023 and is expected to grow to 395.18 million by 2033, indicating its central role in vaccine production.
Rubella Vaccine Human Diploid Cell Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Rubella Vaccine Human Diploid Cell Industry
Merck & Co., Inc.:
Known for its commitment to research and development, Merck produces the MMR vaccine, which includes rubella as a key component, contributing significantly to global vaccination efforts.Sanofi Pasteur:
A subsidiary of Sanofi, Sanofi Pasteur is a leading vaccine manufacturer that plays a vital role in providing vaccines globally, including those for rubella.GlaxoSmithKline (GSK):
GSK is dedicated to advancing the health of populations through the development of innovative vaccines and is a prominent player in the rubella vaccine market.Pfizer Inc.:
A world leader in the pharmaceutical industry, Pfizer contributes to immunization efforts with its vaccine production ensuring widespread availability of rubella vaccines.We're grateful to work with incredible clients.









FAQs
What is the market size of rubella Vaccine Human Diploid Cell?
The global Rubella Vaccine Human Diploid Cell market size was estimated at 300 million USD in 2023, with a projected CAGR of 5%. This growth reflects the increasing vaccination rates and public health initiatives aimed at controlling rubella.
What are the key market players or companies in this rubella Vaccine Human Diploid Cell industry?
Key players in the rubella vaccine market include major pharmaceutical companies such as Merck & Co., Sanofi Pasteur, GlaxoSmithKline, and CSL Behring. These companies dominate the market due to their extensive research, development capabilities, and distribution networks.
What are the primary factors driving the growth in the rubella Vaccine Human Diploid Cell industry?
Growth in the rubella vaccine market is driven by increasing awareness of immunization, government initiatives for childhood vaccination, growing healthcare expenditure, and advancements in vaccine technology, which enhance efficacy and safety.
Which region is the fastest Growing in the rubella Vaccine Human Diploid Cell?
Currently, Asia Pacific is one of the fastest-growing regions, projected to increase from 59.64 million USD in 2023 to 98.15 million USD by 2033. This growth is fueled by rising populations and expanding healthcare infrastructure.
Does ConsaInsights provide customized market report data for the rubella Vaccine Human Diploid Cell industry?
Yes, ConsaInsights offers customized market report data for the rubella vaccine industry. Clients can request tailored analyses based on specific needs, geographic focus, or particular market segments.
What deliverables can I expect from this rubella Vaccine Human Diploid Cell market research project?
Clients can expect deliverables such as comprehensive market analysis reports, regional market data, competitive landscape overviews, consumer insights, and detailed forecasts on market growth and trends over the projected period.
What are the market trends of rubella Vaccine Human Diploid Cell?
Trends in the rubella vaccine market include a focus on combination vaccines, increased reliance on human diploid cell culture for vaccine development, and a strong push for global vaccination campaigns to eliminate rubella.