Safety Syringes Market Report
Published Date: 31 January 2026 | Report Code: safety-syringes
Safety Syringes Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Safety Syringes market, covering key insights, market size, trends, and forecasts from 2023 to 2033. It aims to offer a clear view of the current market landscape and future potential across various segments and regions.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
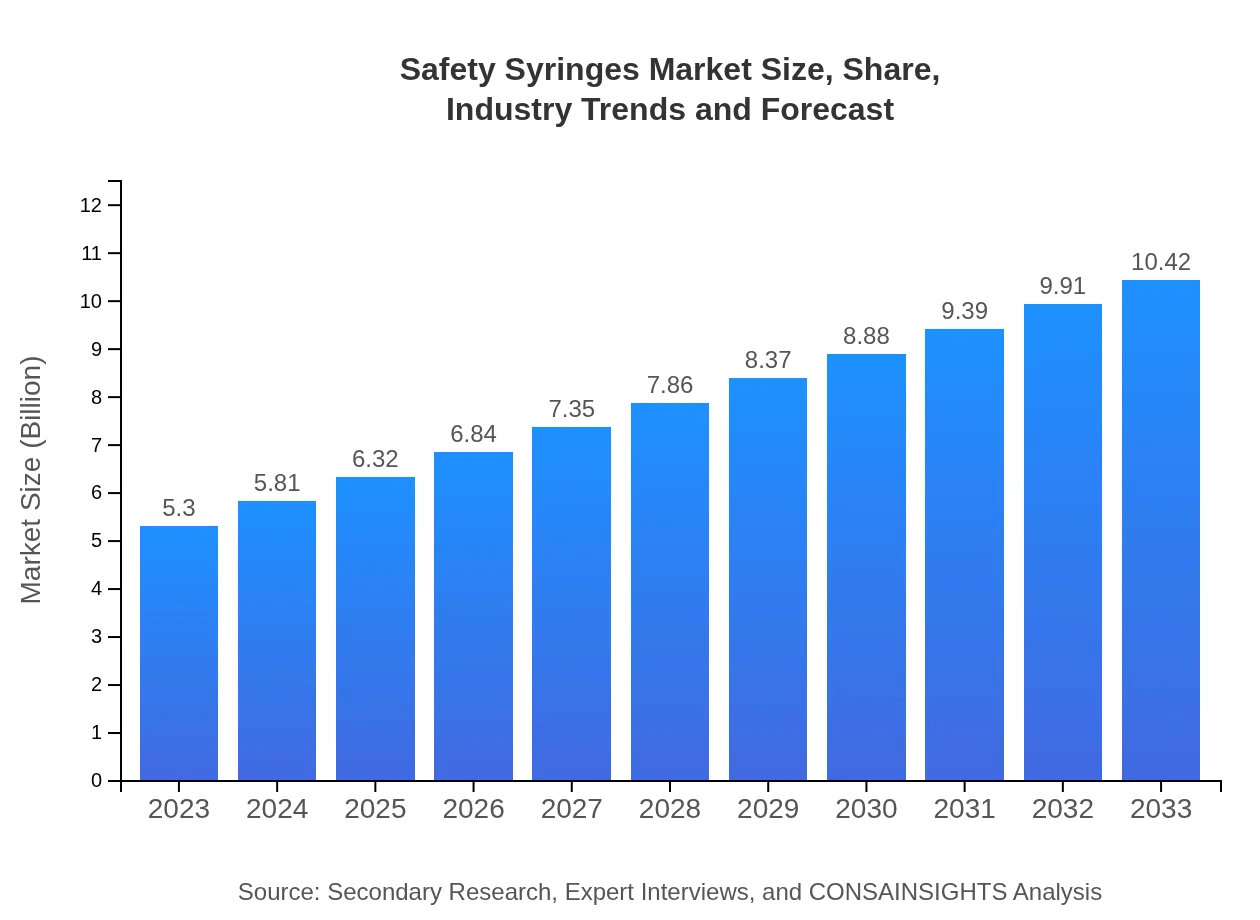
| 2023 Market Size | $5.30 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $10.42 Billion |
| Top Companies | Becton, Dickinson and Company (BD), Cardinal Health, Terumo Corporation, Smiths Medical |
| Last Modified Date | 31 January 2026 |
Safety Syringes Market Overview
Customize Safety Syringes Market Report market research report
- ✔ Get in-depth analysis of Safety Syringes market size, growth, and forecasts.
- ✔ Understand Safety Syringes's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Safety Syringes
What is the Market Size & CAGR of Safety Syringes market in 2023?
Safety Syringes Industry Analysis
Safety Syringes Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Safety Syringes Market Analysis Report by Region
Europe Safety Syringes Market Report:
The European market is projected to grow from $1.57 billion in 2023 to $3.08 billion by 2033. A robust healthcare regulatory framework and a proactive approach toward patient safety significantly influence this growth.Asia Pacific Safety Syringes Market Report:
In 2023, the Asia Pacific Safety Syringes market is valued at approximately $1.11 billion and is anticipated to reach $2.19 billion by 2033. Increased healthcare expenditure and rising awareness regarding the benefits of safety syringes drive growth in countries like India and China.North America Safety Syringes Market Report:
North America represents the largest market for Safety Syringes, with an estimated market size of $1.72 billion in 2023 and a projected growth to $3.39 billion by 2033. The high prevalence of chronic diseases necessitating frequent injections, along with stringent regulations on safety, are key growth factors.South America Safety Syringes Market Report:
The South American Safety Syringes market will grow from $0.52 billion in 2023 to an estimated $1.03 billion by 2033. The region's growth is fueled by an expanding healthcare sector and rising government initiatives for vaccination and public health education.Middle East & Africa Safety Syringes Market Report:
The Middle East and Africa Safety Syringes market is expected to experience growth from $0.37 billion in 2023 to approximately $0.73 billion by 2033. Rising health awareness and improving healthcare infrastructure play crucial roles in market development.Tell us your focus area and get a customized research report.
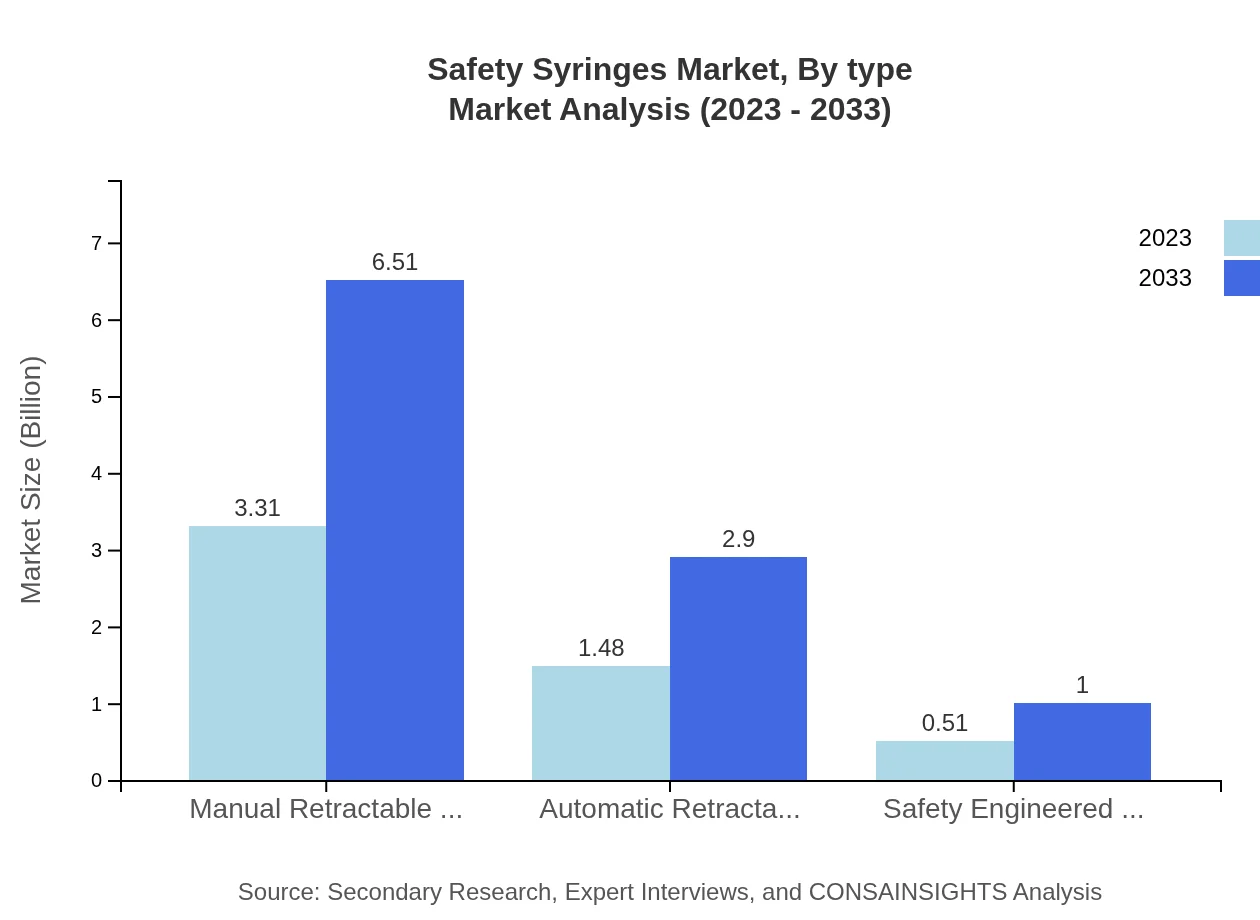
Safety Syringes Market Analysis By Type
The Safety Syringes market by type includes manual retractable syringes with a market size of $3.31 billion in 2023, expected to grow to $6.51 billion by 2033. Automatic retractable syringes and safety-engineered syringes follow with estimates of $1.48 billion and $0.51 billion, respectively, growing towards $2.90 billion and $1 billion in the same period.
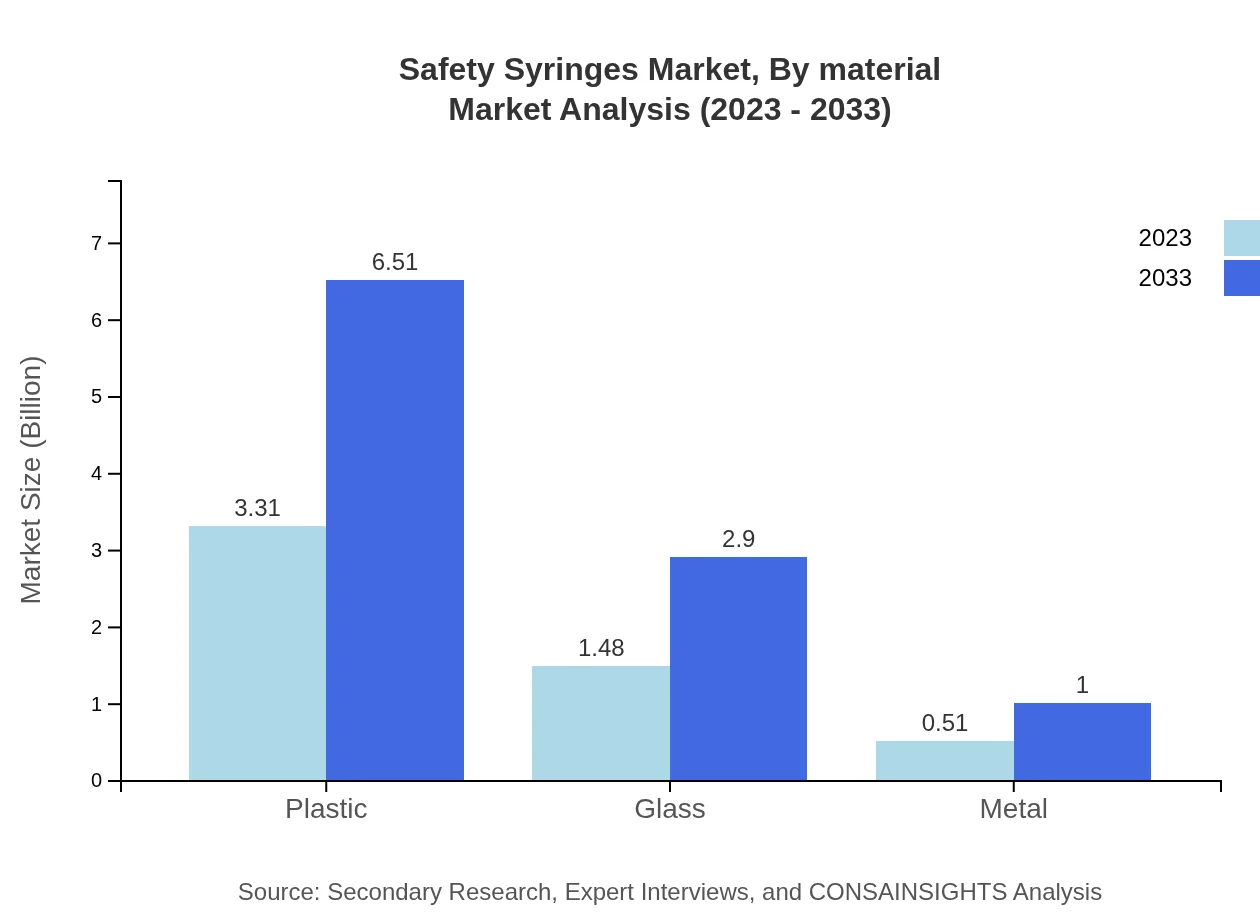
Safety Syringes Market Analysis By Material
Market segmentation by material shows that plastic syringes dominate with a significant market share. They start at $3.31 billion in 2023 and are projected to reach $6.51 billion in 2033, while glass and metal syringes hold smaller segments projected to grow to $2.90 billion and $1 billion, respectively.
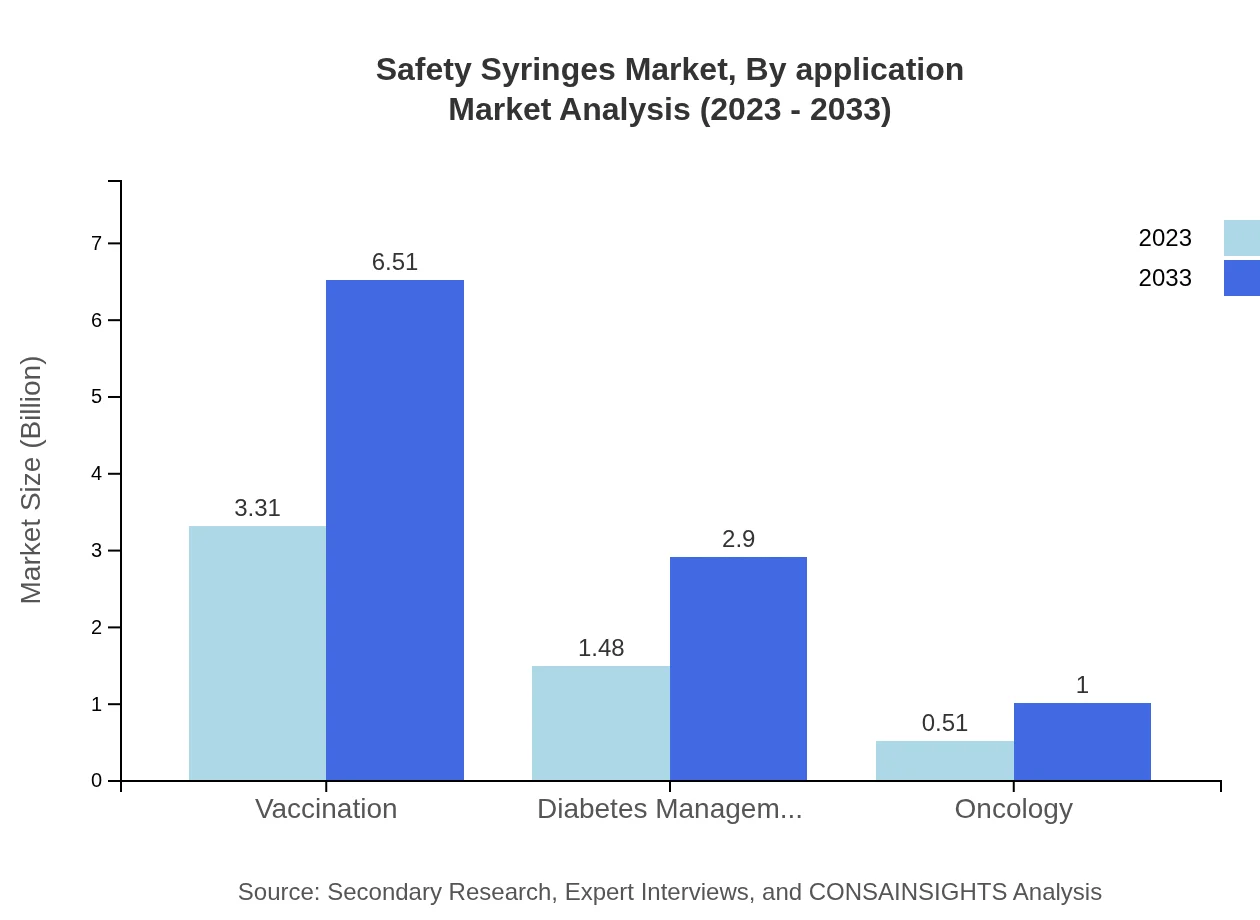
Safety Syringes Market Analysis By Application
In terms of application, vaccination syringes currently represent a substantial share with a market size of $3.31 billion in 2023, anticipated to rise to $6.51 billion by 2033. Other applications such as diabetes management and oncology show similar growth patterns.
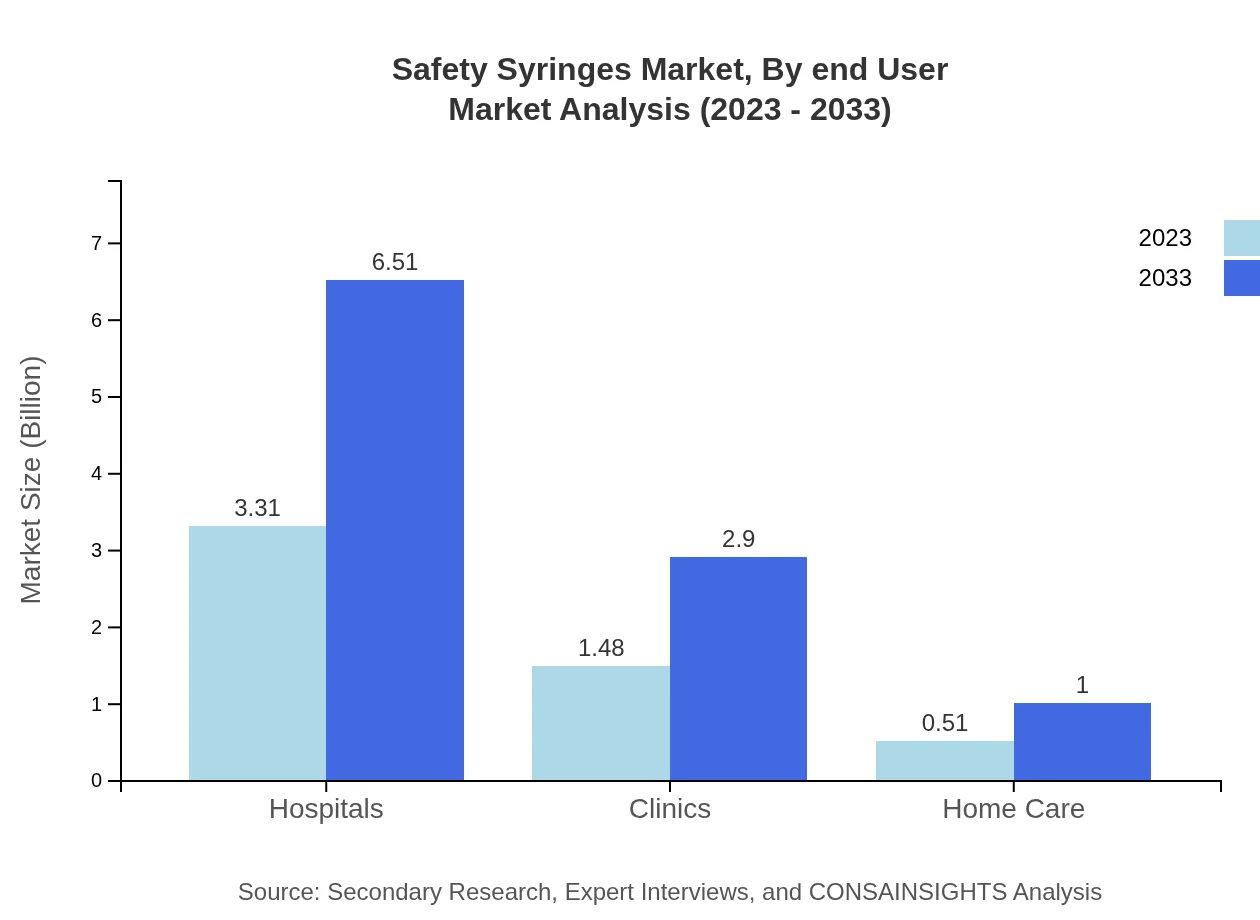
Safety Syringes Market Analysis By End User
Hospitals are the largest segment in the end-user category, valued at $3.31 billion in 2023 and expected to grow to $6.51 billion by 2033. Clinics and home care settings also demonstrate significant growth potential.
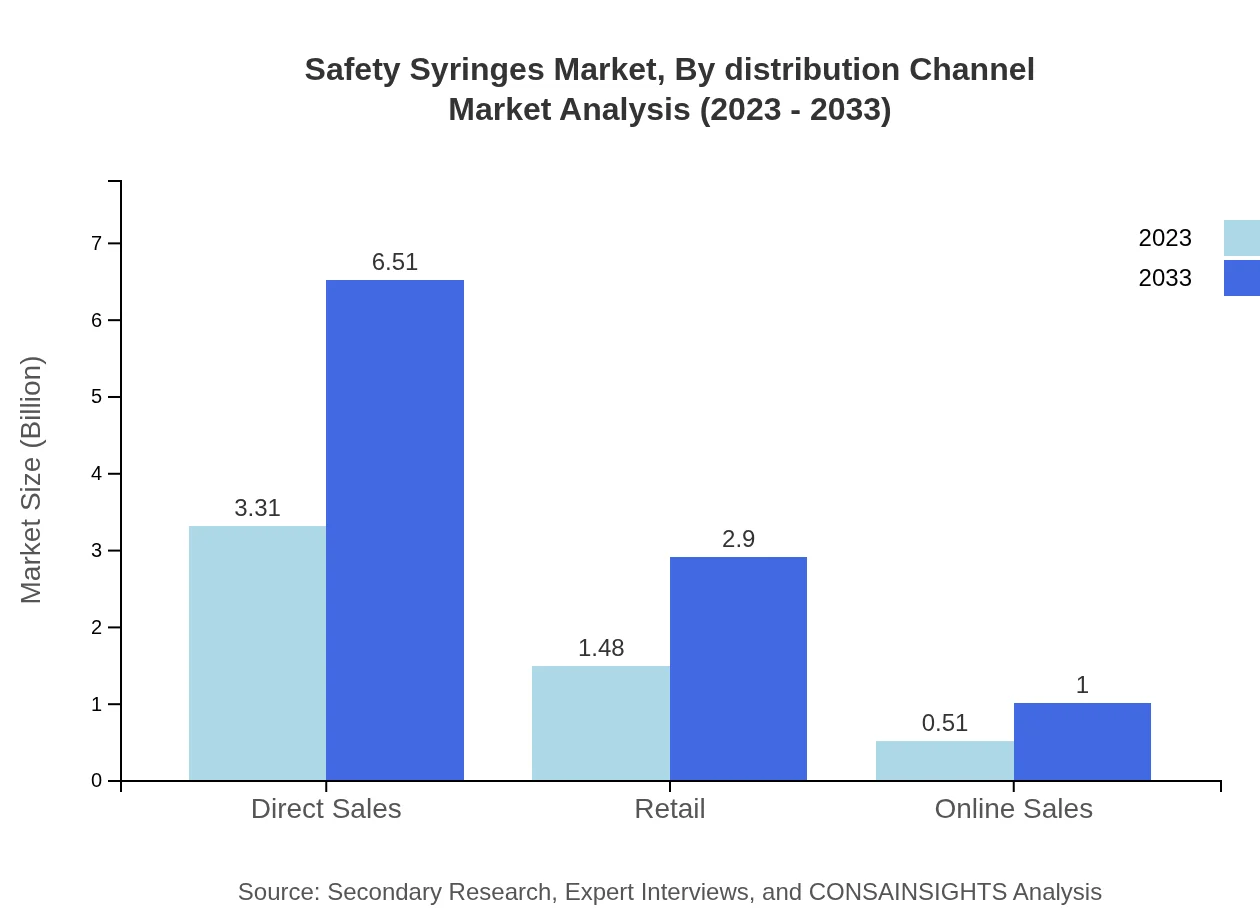
Safety Syringes Market Analysis By Distribution Channel
Direct sales remain the predominant distribution channel with a market value of $3.31 billion in 2023, likely reaching $6.51 billion by 2033. Retail and online sales channels are following closely, driven by increasing online healthcare solutions.
Safety Syringes Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Safety Syringes Industry
Becton, Dickinson and Company (BD):
A leading global medical technology company known for its innovative and high-quality safety syringe solutions contributing significantly to the market.Cardinal Health:
Specializing in healthcare services and products, Cardinal Health focuses on safety-enhanced syringes and has a strong position in the U.S. market.Terumo Corporation:
A major player in the pharmaceutical delivery device sector, Terumo provides a range of safety syringes with advanced safety features.Smiths Medical:
Known for developing innovative medical devices, Smiths Medical has a broad portfolio that includes safety syringes designed to prevent needlestick injuries.We're grateful to work with incredible clients.









FAQs
What is the market size of safety Syringes?
The safety syringes market is currently valued at approximately $5.3 billion, with a projected growth rate of 6.8% CAGR from 2023 to 2033, indicating significant growth and a broadening market presence over the next decade.
What are the key market players or companies in the safety Syringes industry?
Key players in the safety syringes market include major medical device manufacturers such as BD, Terumo Corporation, and Covidien, which are instrumental in driving innovation and compliance in safety syringe design and production.
What are the primary factors driving the growth in the safety Syringes industry?
Growth in the safety syringes industry is primarily driven by increasing awareness of health risks associated with needle-stick injuries, rising incidences of infectious diseases, and regulatory initiatives promoting the use of safety-engineered devices.
Which region is the fastest Growing in the safety Syringes?
The fastest-growing region in the safety syringes market is expected to be North America, where market size is projected to increase from $1.72 billion in 2023 to $3.39 billion by 2033, spurred by high demand in healthcare facilities.
Does ConsaInsights provide customized market report data for the safety Syringes industry?
Yes, ConsaInsights offers customized market report data tailored to specific client needs in the safety syringes industry, enabling clients to access detailed insights across various segments and geographical markets.
What deliverables can I expect from this safety Syringes market research project?
Clients can expect comprehensive deliverables including market analysis reports, growth forecasts, competitor benchmarking data, consumer insights, and strategic recommendations for entering or expanding in the safety syringes market.
What are the market trends of safety Syringes?
Key market trends in the safety syringes sector include a rising preference for manual retractable safety syringes, increasing investments in healthcare infrastructure, and evolving consumer demand for safety in medical devices.