Scleroderma Diagnostics And Therapeutics Market Report
Published Date: 31 January 2026 | Report Code: scleroderma-diagnostics-and-therapeutics
Scleroderma Diagnostics And Therapeutics Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Scleroderma Diagnostics and Therapeutics market, detailing current conditions, market size, competitive landscape, and growth forecasts from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
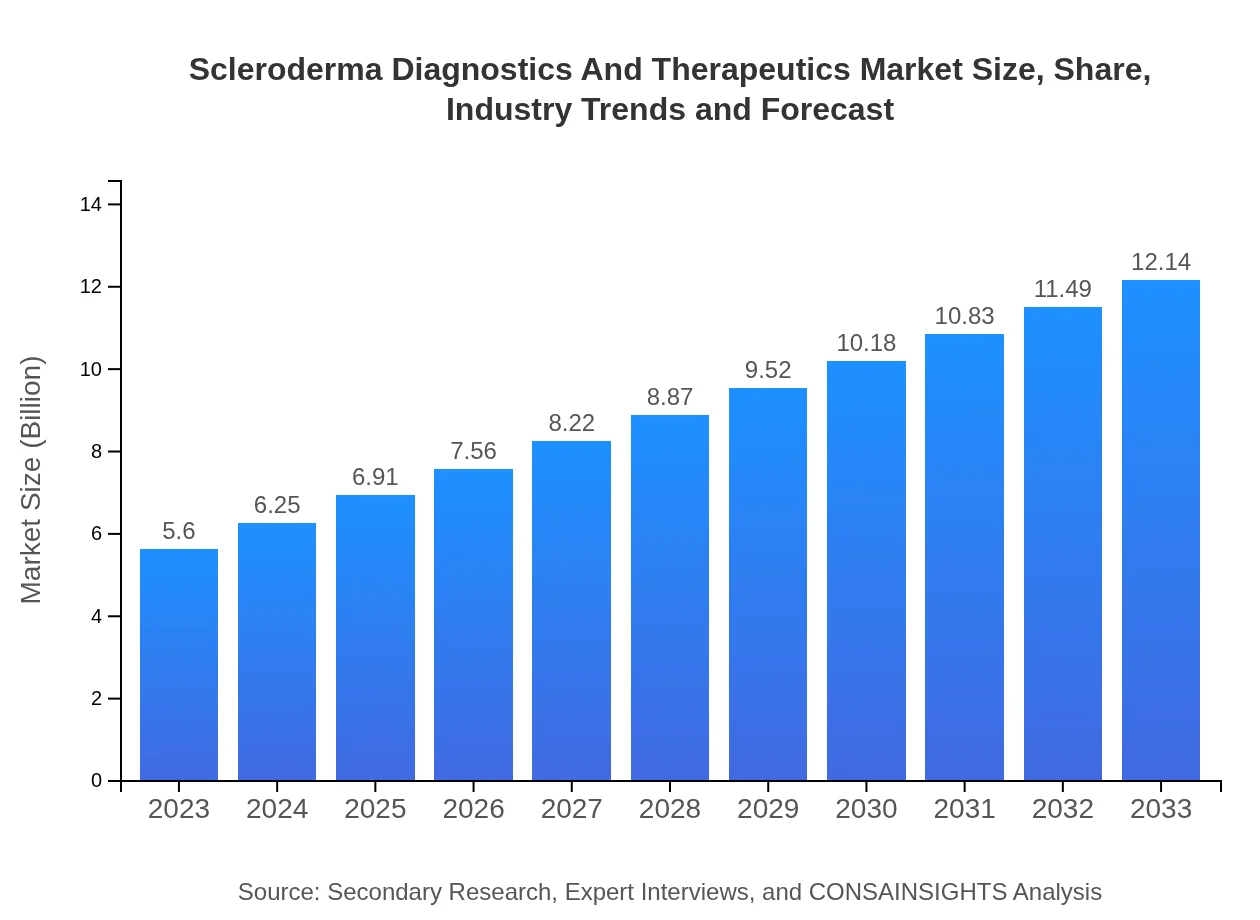
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 7.8% |
| 2033 Market Size | $12.14 Billion |
| Top Companies | Roche, Novartis, Bristol-Myers Squibb, Merck & Co. |
| Last Modified Date | 31 January 2026 |
Scleroderma Diagnostics And Therapeutics Market Overview
Customize Scleroderma Diagnostics And Therapeutics Market Report market research report
- ✔ Get in-depth analysis of Scleroderma Diagnostics And Therapeutics market size, growth, and forecasts.
- ✔ Understand Scleroderma Diagnostics And Therapeutics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Scleroderma Diagnostics And Therapeutics
What is the Market Size & CAGR of Scleroderma Diagnostics And Therapeutics market in 2023?
Scleroderma Diagnostics And Therapeutics Industry Analysis
Scleroderma Diagnostics And Therapeutics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Scleroderma Diagnostics And Therapeutics Market Analysis Report by Region
Europe Scleroderma Diagnostics And Therapeutics Market Report:
Europe's market is expected to grow from $1.79 billion in 2023 to $3.88 billion by 2033, underpinned by stringent regulatory frameworks encouraging innovation and an increase in healthcare investments across member states.Asia Pacific Scleroderma Diagnostics And Therapeutics Market Report:
The Asia Pacific region is expected to grow from $0.95 billion in 2023 to $2.06 billion by 2033, favorably impacted by an increasing patient pool, growing healthcare expenditure, and expanding access to diagnostics and treatment options.North America Scleroderma Diagnostics And Therapeutics Market Report:
North America dominates the market with a size of $2.03 billion in 2023, anticipated to reach $4.40 billion in 2033. The growth is fueled by advanced healthcare systems, significant research funding, and a robust pipeline of innovative therapies.South America Scleroderma Diagnostics And Therapeutics Market Report:
In South America, the market is projected to expand from $0.38 billion in 2023 to $0.82 billion by 2033, driven largely by heightened awareness of scleroderma and investment in healthcare infrastructure.Middle East & Africa Scleroderma Diagnostics And Therapeutics Market Report:
The Middle East and Africa are projected to increase from $0.45 billion in 2023 to $0.98 billion by 2033, reflecting improvements in healthcare access and increasing awareness of the disease.Tell us your focus area and get a customized research report.
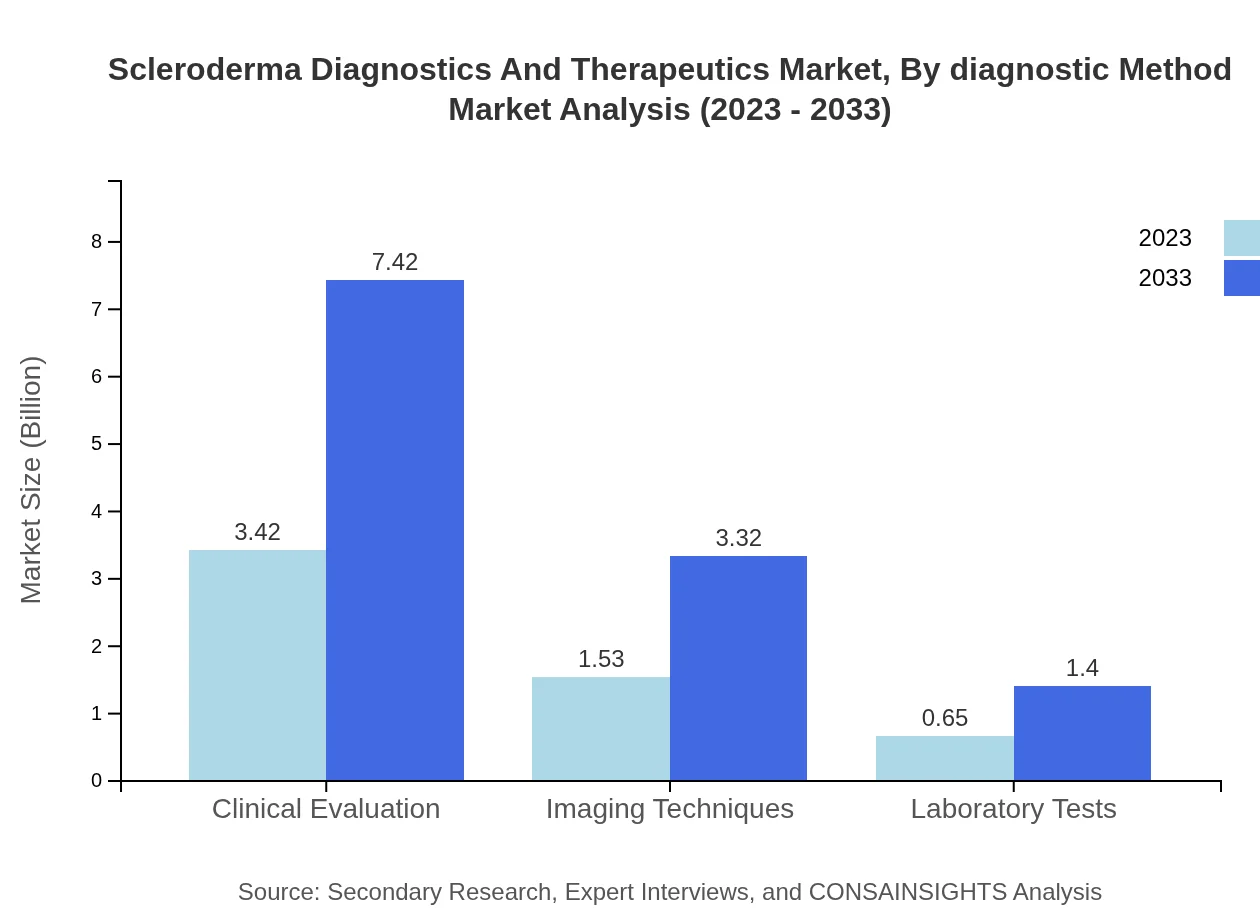
Scleroderma Diagnostics And Therapeutics Market Analysis By Diagnostic Method
The diagnostics segment includes Clinical Evaluation, Imaging Techniques, and Laboratory Tests. In 2023, Clinical Evaluation leads with a market size of $3.42 billion, capturing 61.09%. Imaging Techniques follow with $1.53 billion (27.34%), and Laboratory Tests hold $0.65 billion (11.57%). The increasing trend towards non-invasive diagnostic approaches is enhancing the focus on imaging technologies.
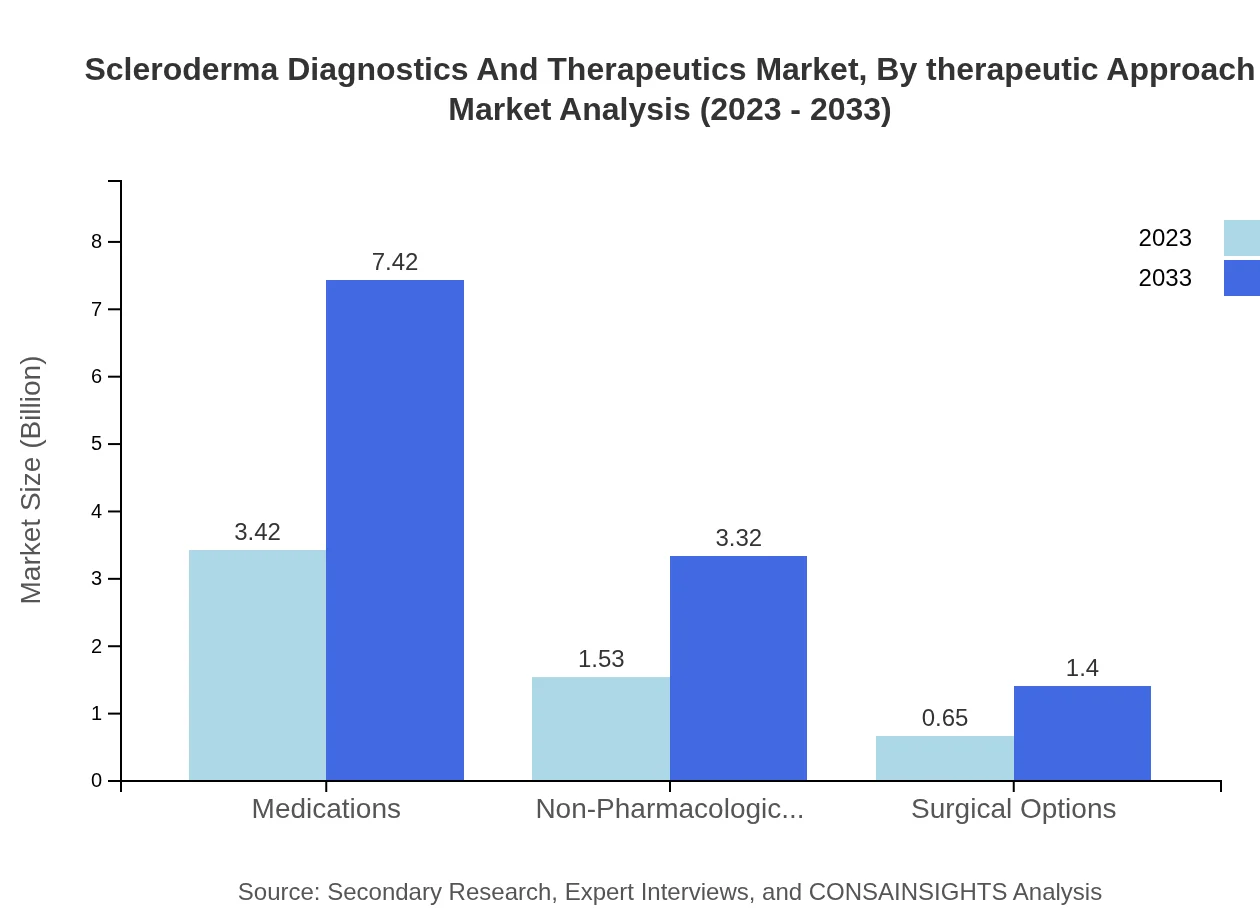
Scleroderma Diagnostics And Therapeutics Market Analysis By Therapeutic Approach
Regarding therapeutic approaches, Medications dominate with a size of $3.42 billion (61.09%), followed by Non-Pharmacological Therapies at $1.53 billion (27.34%) and Surgical Options at $0.65 billion (11.57%). This reflects a growing preference for integrated treatment methodologies blending medication and therapy.
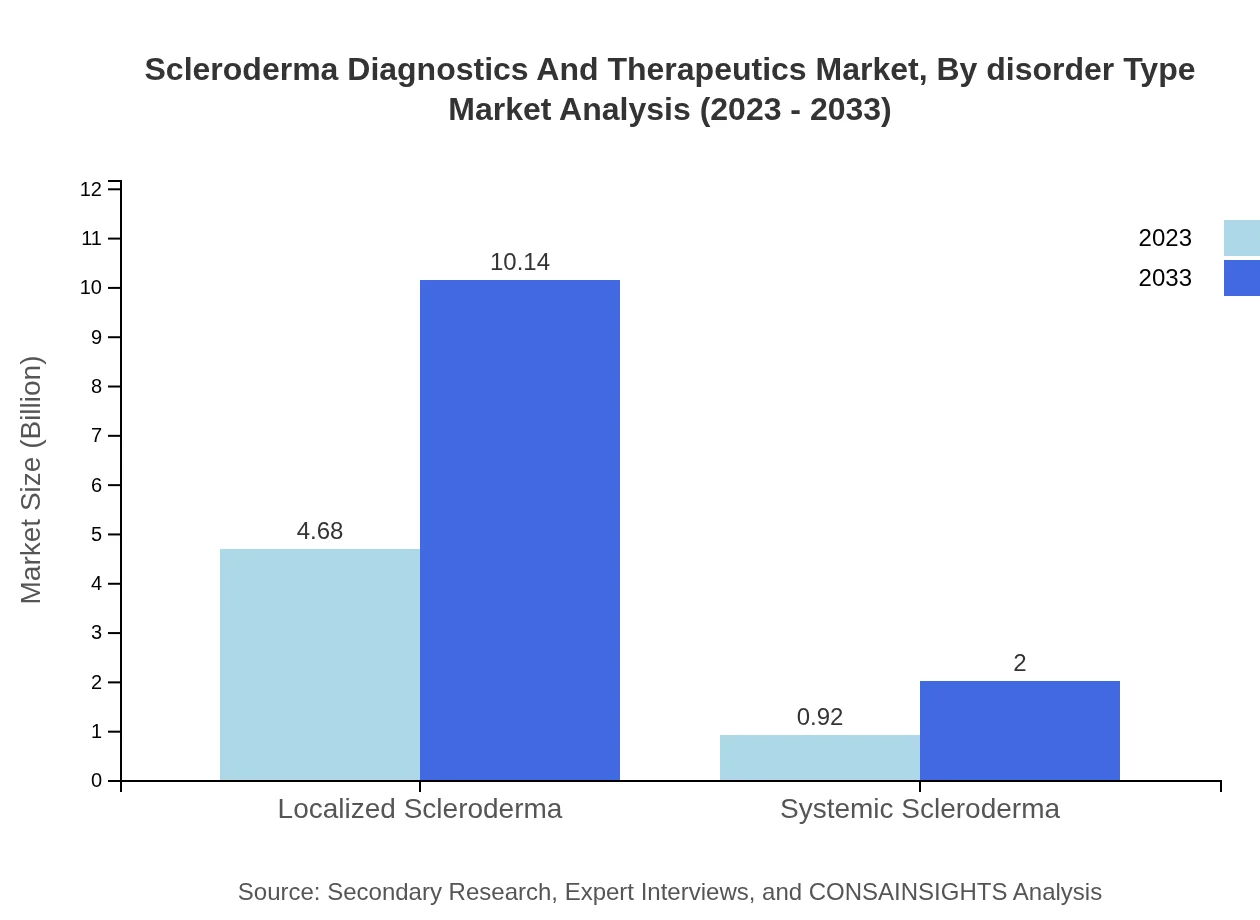
Scleroderma Diagnostics And Therapeutics Market Analysis By Disorder Type
The market segmentation by disorder type indicates that Localized Scleroderma accounts for $4.68 billion (83.49%) in 2023, contrasting with Systemic Scleroderma at $0.92 billion (16.51%). Continuous research into bespoke treatments for systemic scleroderma is likely to drive increased market share in the coming years.
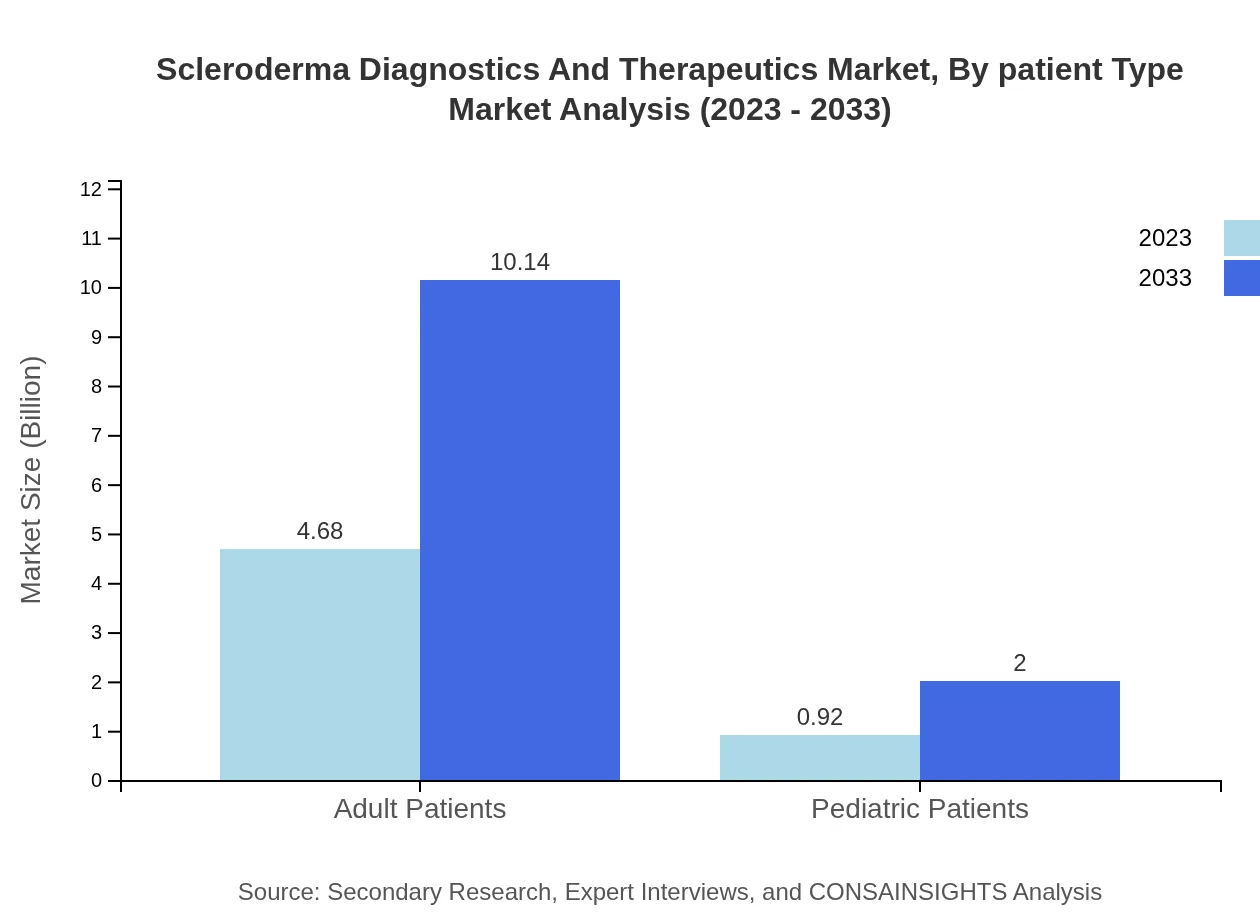
Scleroderma Diagnostics And Therapeutics Market Analysis By Patient Type
In patient type segmentation, Adult Patients hold a substantial market size of $4.68 billion (83.49%), while Pediatric Patients account for $0.92 billion (16.51%). This trend emphasizes the need for targeted treatment protocols that cater specifically to both adult and pediatric demographics.
Scleroderma Diagnostics And Therapeutics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Scleroderma Diagnostics And Therapeutics Industry
Roche:
A leader in biotechnology, Roche focuses on innovative treatments and diagnostics in autoimmune diseases, including scleroderma.Novartis:
Pioneering diverse therapeutic solutions, Novartis emphasizes research in scleroderma treatment advancements.Bristol-Myers Squibb:
This biopharmaceutical company is known for its contributions to developing effective treatments for systemic autoimmune diseases.Merck & Co.:
A long-standing pharmaceutical company dedicated to advancing treatment protocols for autoimmune conditions through extensive research and development.We're grateful to work with incredible clients.









FAQs
What is the market size of scleroderma Diagnostics And Therapeutics?
The global market size for scleroderma diagnostics and therapeutics is projected to reach approximately $5.6 billion by 2033, with a compound annual growth rate (CAGR) of 7.8% from 2023 to 2033. This growth reflects increasing awareness and advancements in therapeutic approaches.
What are the key market players or companies in the scleroderma Diagnostics And Therapeutics industry?
The key players in the scleroderma diagnostics and therapeutics industry include major pharmaceutical companies and diagnostic laboratories, focusing on innovative treatment solutions and advanced diagnostic technologies. Their contribution is crucial for the ongoing development of effective scleroderma therapies.
What are the primary factors driving the growth in the scleroderma Diagnostics And Therapeutics industry?
The growth in the scleroderma diagnostics and therapeutics industry is driven by factors such as increasing prevalence of scleroderma, advancements in treatments, and growing acceptance of early diagnostic techniques which enhance patient management and improve quality of life.
Which region is the fastest Growing in the scleroderma Diagnostics And Therapeutics?
The Asia Pacific region is expected to be the fastest-growing market for scleroderma diagnostics and therapeutics, projected to grow from $0.95 billion in 2023 to $2.06 billion by 2033. This growth is attributed to improving healthcare infrastructure and increasing patient awareness.
Does ConsaInsights provide customized market report data for the scleroderma Diagnostics And Therapeutics industry?
Yes, ConsaInsights offers customized market report data tailored to the specific needs of stakeholders in the scleroderma diagnostics and therapeutics industry. These reports provide in-depth analysis and insights for informed decision-making.
What deliverables can I expect from this scleroderma Diagnostics And Therapeutics market research project?
Deliverables from the scleroderma diagnostics and therapeutics market research project typically include detailed market analysis, segment data, regional breakdown, competitive landscape, and expert insights to support strategic planning and investment decisions.
What are the market trends of scleroderma Diagnostics And Therapeutics?
Key market trends include a shift towards personalized medicine, rising focus on innovative diagnostic tools, increased collaborations among key players, and a growing emphasis on patient-centric treatment approaches in the scleroderma diagnostics and therapeutics landscape.