Seasonal Influenza Vaccine Market Report
Published Date: 31 January 2026 | Report Code: seasonal-influenza-vaccine
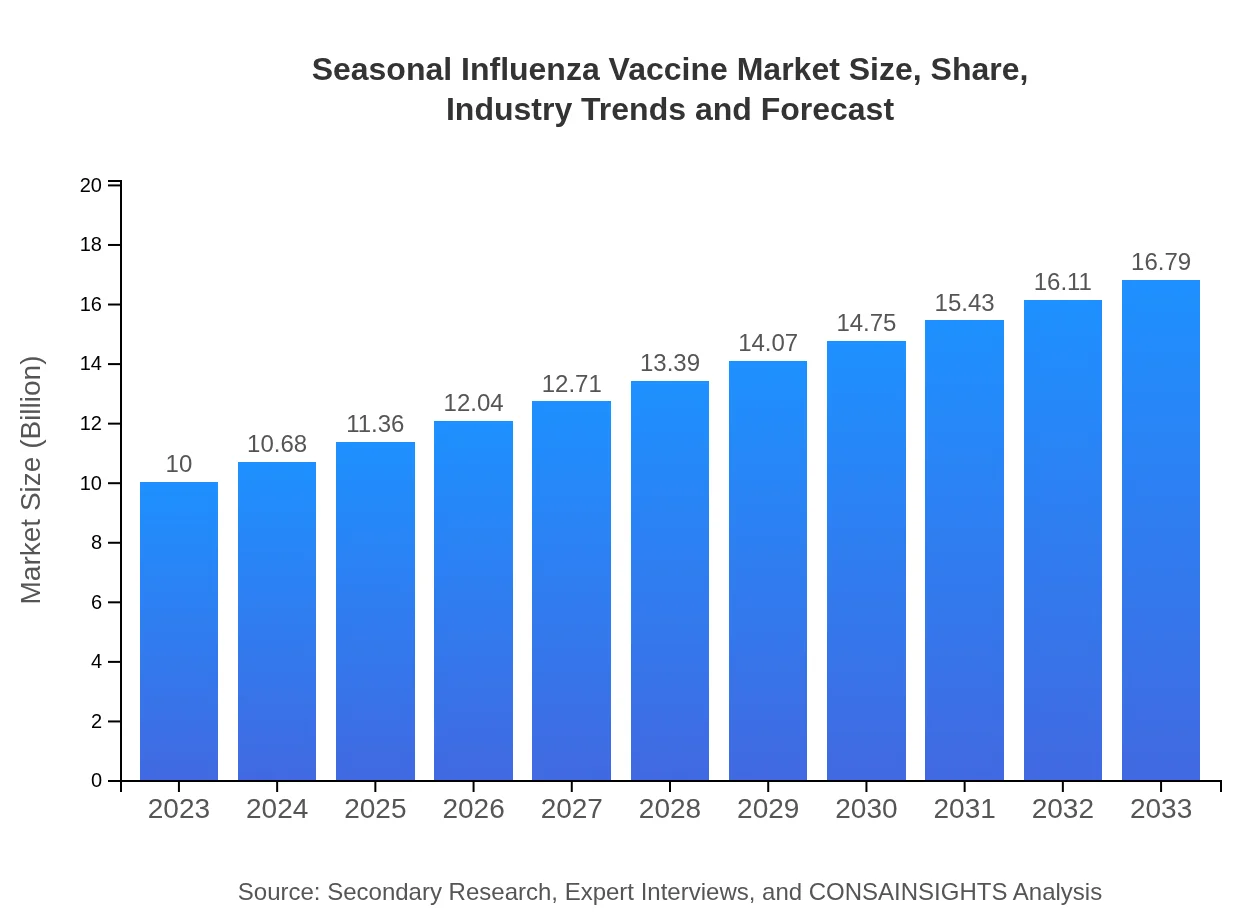
Seasonal Influenza Vaccine Market Size, Share, Industry Trends and Forecast to 2033
This report provides an extensive analysis of the Seasonal Influenza Vaccine market, covering trends, insights, and forecasts from 2023 to 2033. It explores the market size, segmentation, regional breakdown, and key players, offering strategic insights for stakeholders.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $10.00 Billion |
| CAGR (2023-2033) | 5.2% |
| 2033 Market Size | $16.79 Billion |
| Top Companies | Sanofi Pasteur, GlaxoSmithKline plc, ASTRAZENECA, Pfizer , Novartis |
| Last Modified Date | 31 January 2026 |
Seasonal Influenza Vaccine Market Overview
Customize Seasonal Influenza Vaccine Market Report market research report
- ✔ Get in-depth analysis of Seasonal Influenza Vaccine market size, growth, and forecasts.
- ✔ Understand Seasonal Influenza Vaccine's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Seasonal Influenza Vaccine
What is the Market Size & CAGR of Seasonal Influenza Vaccine market in 2023?
Seasonal Influenza Vaccine Industry Analysis
Seasonal Influenza Vaccine Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Seasonal Influenza Vaccine Market Analysis Report by Region
Europe Seasonal Influenza Vaccine Market Report:
The European market for Seasonal Influenza Vaccines is anticipated to grow from $3.10 billion in 2023 to approximately $5.21 billion by 2033. European governments emphasize vaccination policies and initiatives, which encourage higher uptake rates and drive market expansion.Asia Pacific Seasonal Influenza Vaccine Market Report:
The Asia Pacific region plays a significant role in the Seasonal Influenza Vaccine market, exhibiting strong growth from $1.88 billion in 2023 to an estimated $3.15 billion in 2033. Increased healthcare expenditure, government initiatives for vaccination programs, and an uptick in influenza cases are key factors driving investments in this region.North America Seasonal Influenza Vaccine Market Report:
North America remains the largest market for Seasonal Influenza Vaccines, with a size of $3.70 billion in 2023, expected to grow to $6.21 billion by 2033. This growth is driven by high vaccination rates, robust healthcare infrastructure, and large-scale government vaccination campaigns. The US holds a dominant position due to its comprehensive immunization programs.South America Seasonal Influenza Vaccine Market Report:
In South America, the market is gradually evolving, valued at $0.46 billion in 2023 and projected to reach $0.78 billion by 2033. The region sees challenges in logistics and cold chain management but benefits from rising awareness and vaccination initiatives supported by international health organizations.Middle East & Africa Seasonal Influenza Vaccine Market Report:
In the Middle East and Africa, the market is expected to grow from $0.86 billion in 2023 to $1.44 billion in 2033. The region faces infrastructural challenges but shows promise due to increasing healthcare investments and rising awareness about infectious diseases.Tell us your focus area and get a customized research report.
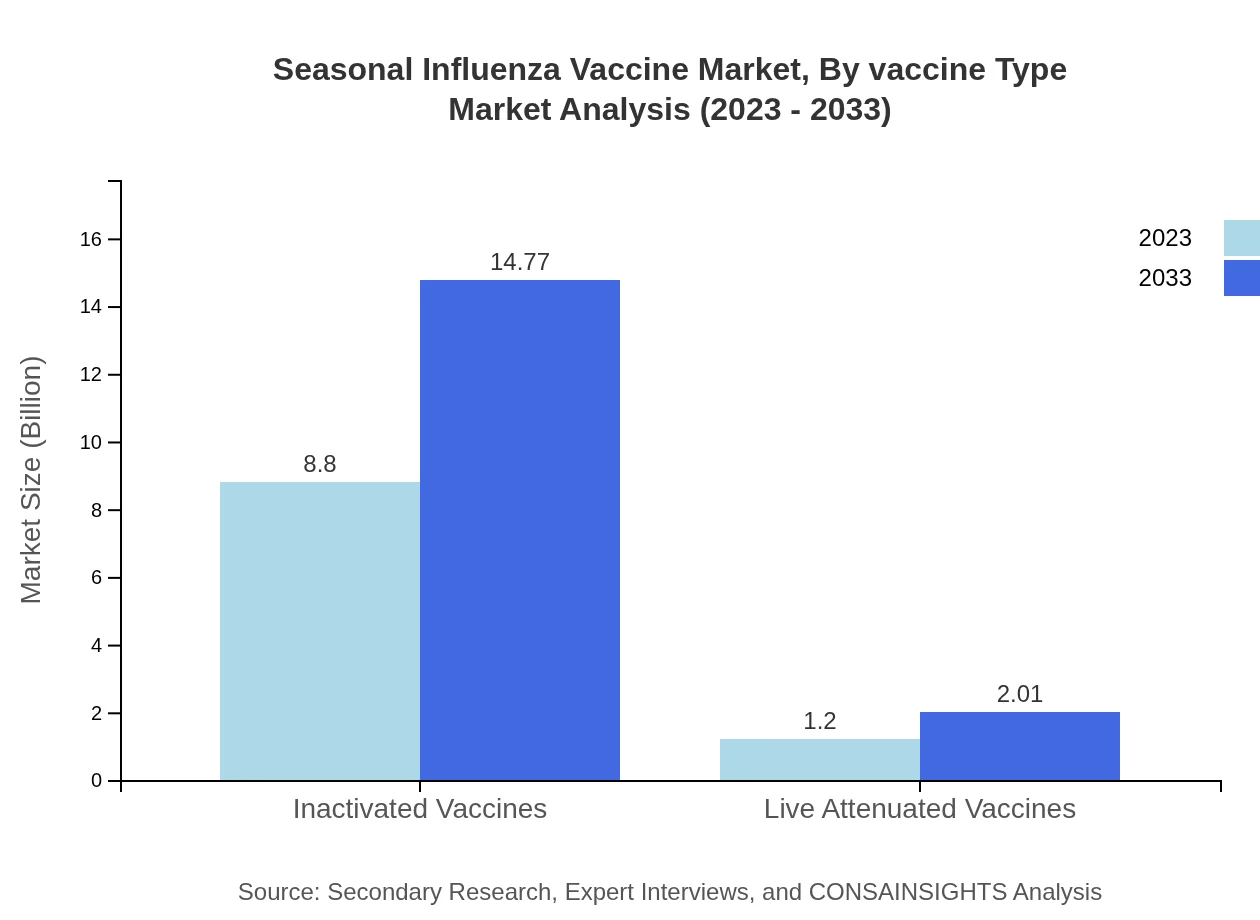
Seasonal Influenza Vaccine Market Analysis By Vaccine Type
The Seasonal Influenza Vaccine market by vaccine type is largely dominated by inactivated vaccines, holding an 88.01% share in 2023 with a projected size of $14.77 billion, increasing to 14.77 billion by 2033. Live attenuated vaccines follow, making up 11.99% of the market share, while recombinant technologies represent a smaller niche but growing segment, marking $0.84 billion in 2023.
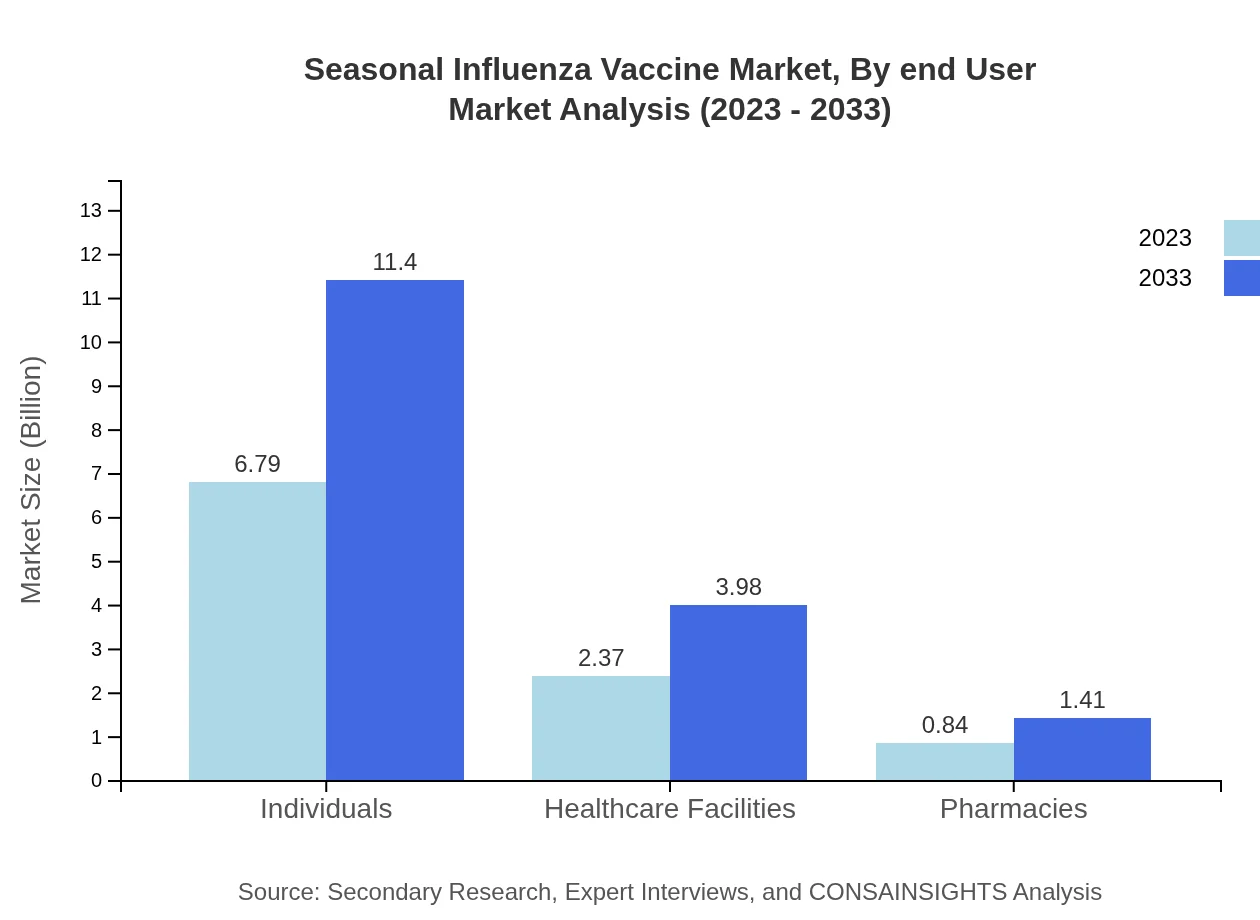
Seasonal Influenza Vaccine Market Analysis By End User
Individuals constitute the largest segment of the Seasonal Influenza Vaccine market, with a size of $6.79 billion in 2023 and expected to reach $11.40 billion by 2033, highlighting the importance of public awareness campaigns. Healthcare facilities represent 23.71% of the market share, while pharmacies, though smaller, contribute to accessibility and awareness.
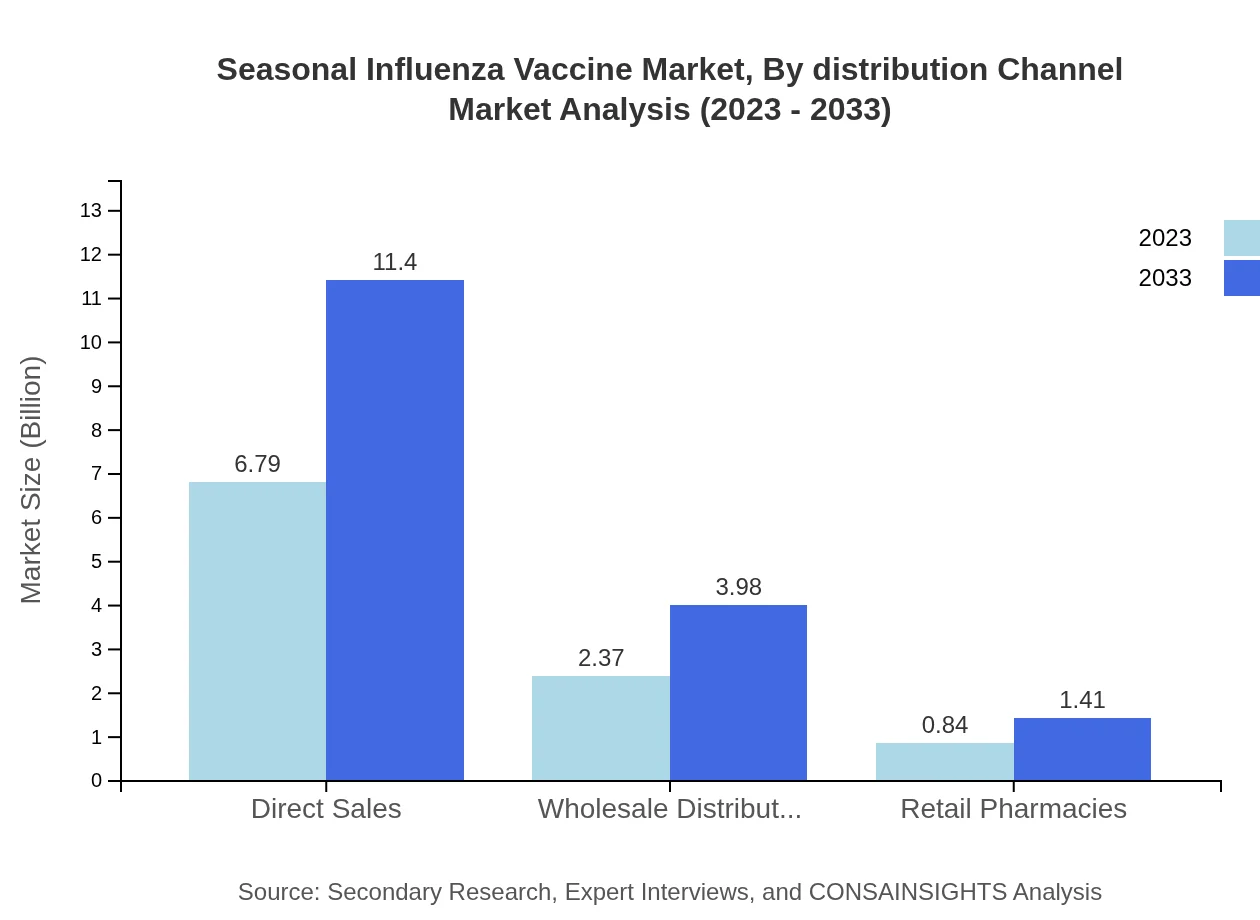
Seasonal Influenza Vaccine Market Analysis By Distribution Channel
Direct sales dominate the distribution channels, accounting for 67.92% of the market in 2023. Although less dominant, retail pharmacies and wholesale distributors play essential roles in making vaccines accessible, contributing to overall market growth as direct sales and pharmacy sales increase steadily.
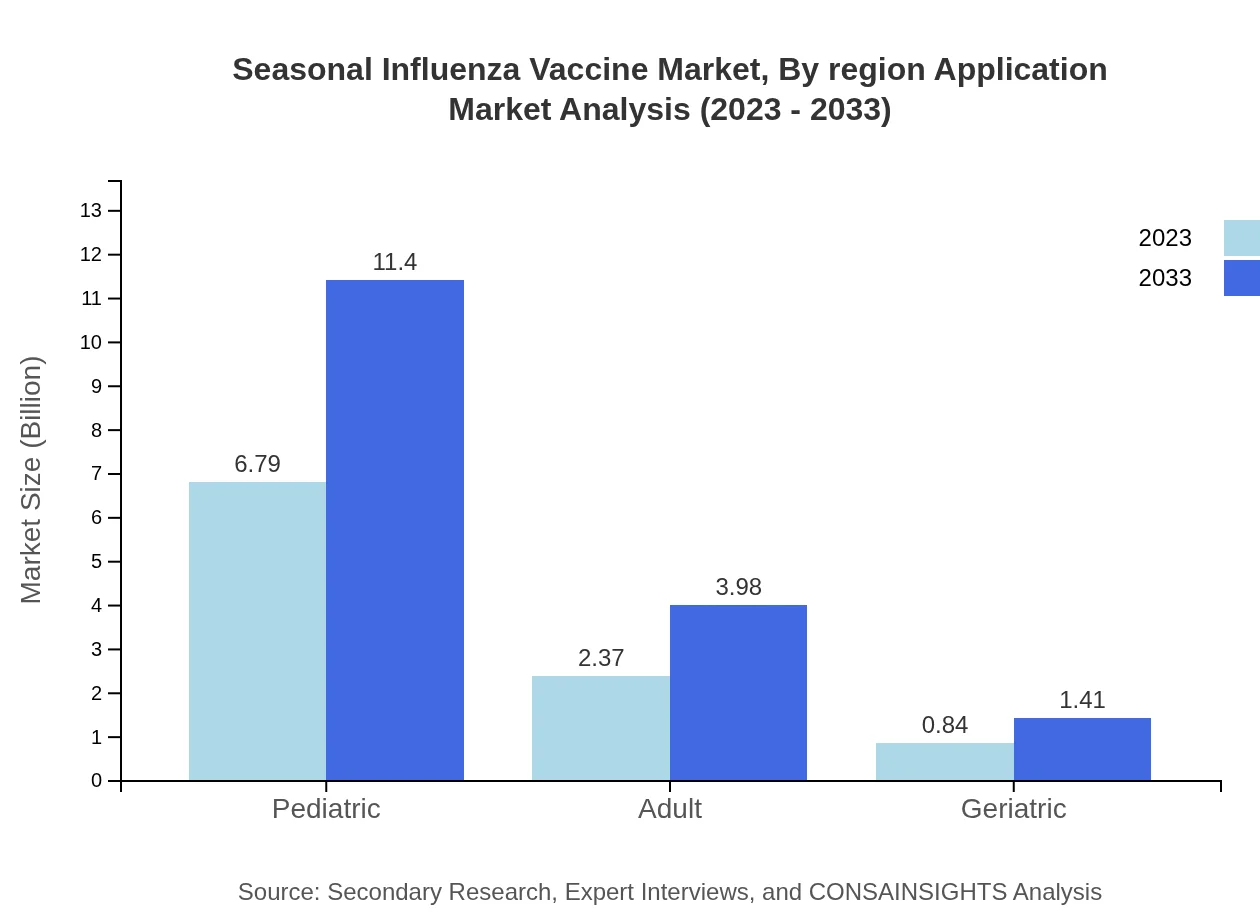
Seasonal Influenza Vaccine Market Analysis By Region Application
The pediatric segment accounts for a significant portion of the Seasonal Influenza Vaccine market, demonstrating the importance of immunization in children with a share of 67.92% as of 2023. Adult and geriatric sectors are growing but represent smaller segments of the market, which will continue to see increased allocations as geriatric populations expand.
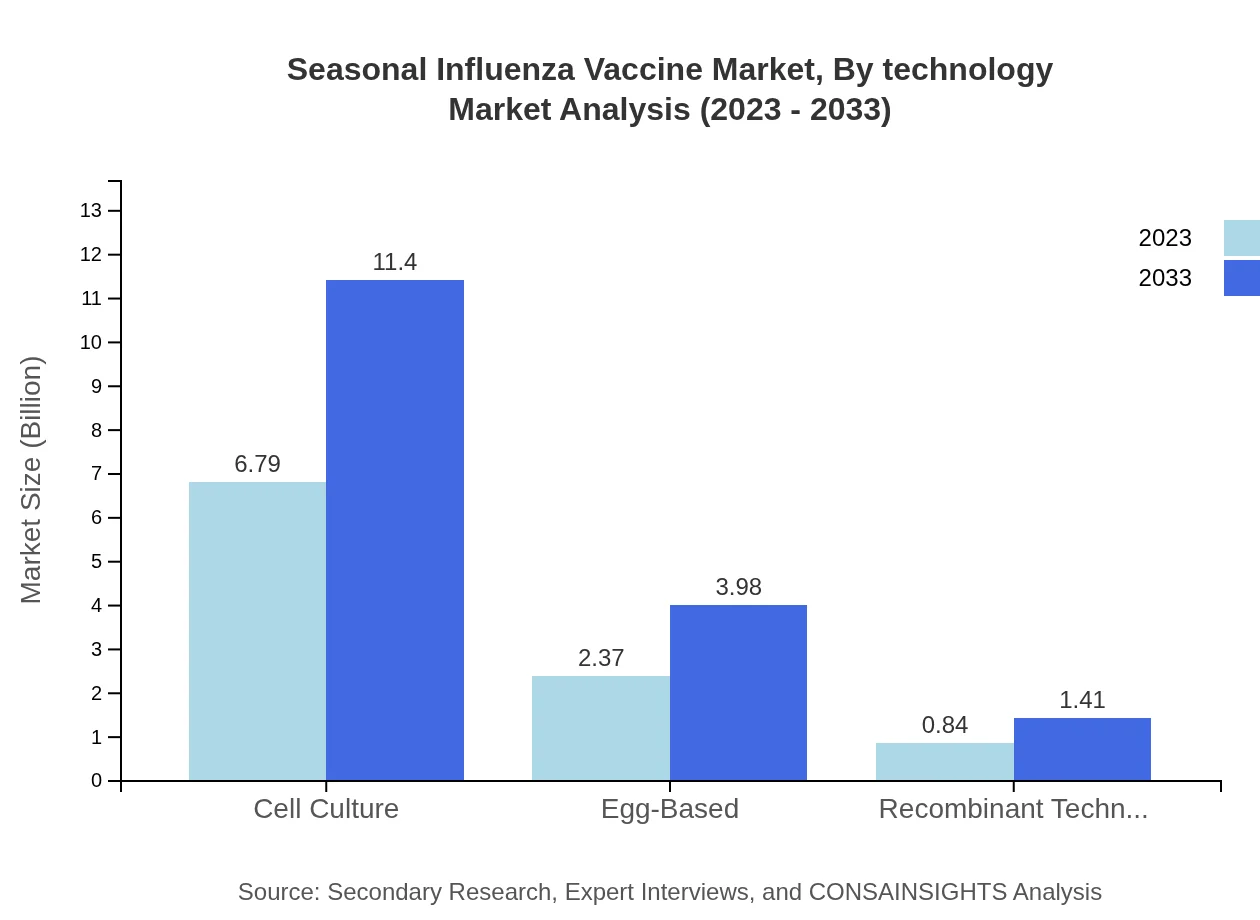
Seasonal Influenza Vaccine Market Analysis By Technology
Cell culture technology comprises the largest share within the Seasonal Influenza Vaccine market, holding a substantial 67.92% share in 2023. Egg-based technology still holds importance, especially in traditional manufacturing processes, but recombinant technology is catching up, offering modern solutions and increasing interest from healthcare providers.
Seasonal Influenza Vaccine Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Seasonal Influenza Vaccine Industry
Sanofi Pasteur:
A leading manufacturer, Sanofi Pasteur develops and distributes seasonal influenza vaccines globally, focusing on innovative solutions that enhance efficacy.GlaxoSmithKline plc:
GSK is renowned for its commitment to research in influenza vaccines, continuously investing in advanced technologies to improve patient outcomes.ASTRAZENECA:
AstraZeneca provides a broad range of vaccines and focuses on enhancing vaccine accessibility while ensuring high safety and efficacy standards.Pfizer :
Pfizer is a key player in the flu vaccine market, recognized for developing advanced formulations and expanding vaccine availability worldwide.Novartis:
With a focus on research and quality, Novartis remains a strong contributor to the seasonal influenza vaccine market, providing effective products.We're grateful to work with incredible clients.









FAQs
What is the market size of seasonal Influenza Vaccine?
The seasonal influenza vaccine market is projected to reach approximately $10 billion by 2033, with a CAGR of 5.2% from 2023. This growth is fueled by increasing vaccination rates and awareness of seasonal influenza's risks.
What are the key market players or companies in this seasonal Influenza Vaccine industry?
Key players in the seasonal influenza vaccine industry include major pharmaceutical companies such as Sanofi Pasteur, GlaxoSmithKline, AstraZeneca, and Merck. These companies play a significant role in research, manufacturing, and distribution of vaccines globally.
What are the primary factors driving the growth in the seasonal Influenza Vaccine industry?
Factors driving growth in the seasonal influenza vaccine market include rising awareness about influenza disease, increased healthcare spending, government vaccination programs, and technological advancements in vaccine development and storage.
Which region is the fastest Growing in the seasonal Influenza Vaccine?
North America is the fastest-growing region in the seasonal influenza vaccine market, projected to grow from $3.70 billion in 2023 to $6.21 billion by 2033. The increasing healthcare infrastructure and vaccination initiatives are major contributors.
Does ConsaInsights provide customized market report data for the seasonal Influenza Vaccine industry?
Yes, ConsaInsights offers customized market report data tailored to the seasonal influenza vaccine industry. These reports can be adjusted to focus on specific regions, segments, and company profiles as per client requirements.
What deliverables can I expect from this seasonal Influenza Vaccine market research project?
From the seasonal influenza vaccine market research project, you can expect detailed market analysis, trend forecasting, competitive landscape assessments, regional insights, and segmentation data encompassing demographics and distribution channels.
What are the market trends of seasonal Influenza Vaccine?
Current market trends in the seasonal influenza vaccine sector highlight the transition towards cell culture-based and recombinant technologies, increased adoption of inactivated vaccines, and an emphasis on pediatric offerings to enhance overall vaccination rates.