Sepsis Therapeutics Market Report
Published Date: 31 January 2026 | Report Code: sepsis-therapeutics
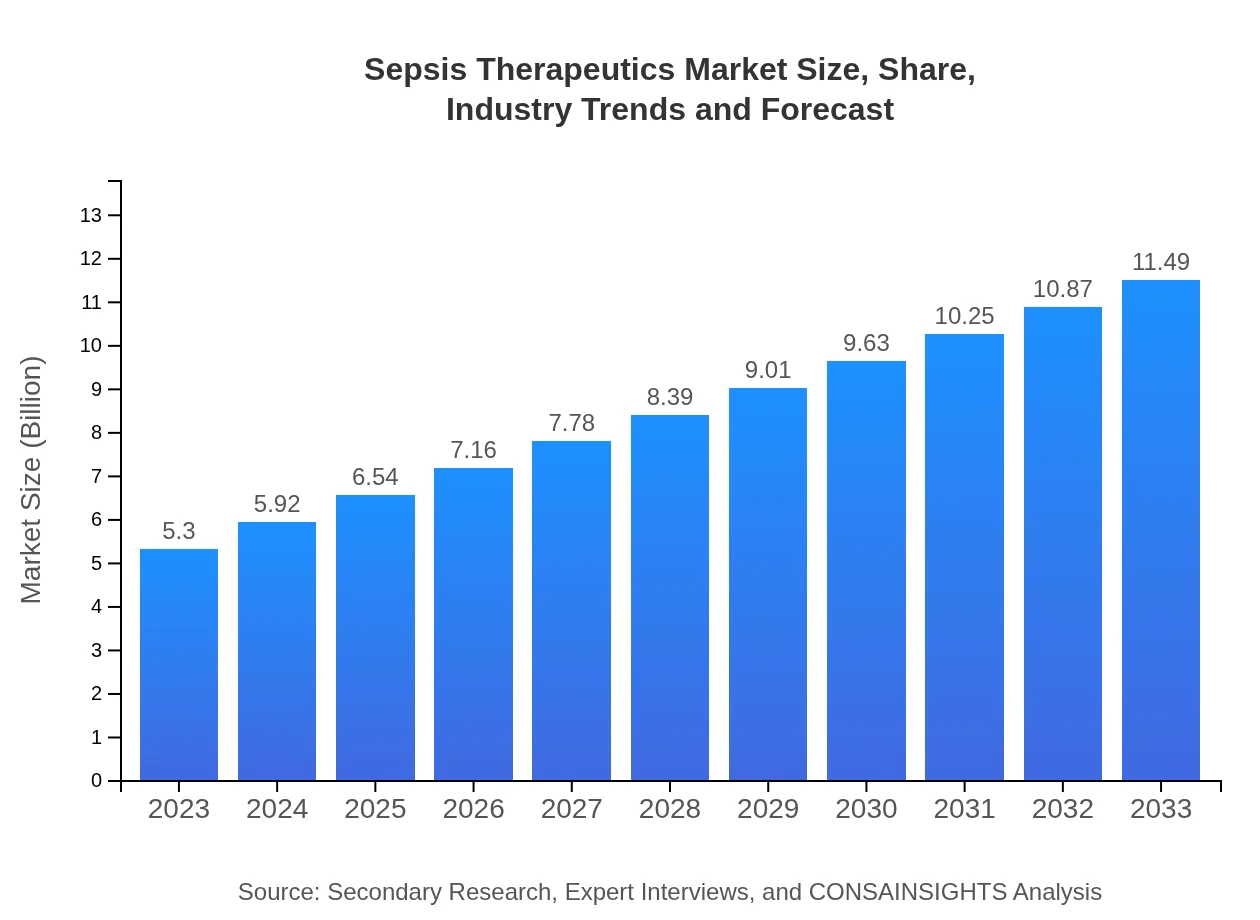
Sepsis Therapeutics Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Sepsis Therapeutics market, including current trends, market dynamics, segmentation, and regional insights. It also outlines market forecasts from 2023 to 2033, offering valuable data for stakeholders looking to navigate the evolving landscape of sepsis treatment.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $5.30 Billion |
| CAGR (2023-2033) | 7.8% |
| 2033 Market Size | $11.49 Billion |
| Top Companies | Pfizer , Novo Nordisk, AstraZeneca, Merck & Co., GlaxoSmithKline |
| Last Modified Date | 31 January 2026 |
Sepsis Therapeutics Market Overview
Customize Sepsis Therapeutics Market Report market research report
- ✔ Get in-depth analysis of Sepsis Therapeutics market size, growth, and forecasts.
- ✔ Understand Sepsis Therapeutics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Sepsis Therapeutics
What is the Market Size & CAGR of Sepsis Therapeutics market in 2023?
Sepsis Therapeutics Industry Analysis
Sepsis Therapeutics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Sepsis Therapeutics Market Analysis Report by Region
Europe Sepsis Therapeutics Market Report:
In Europe, the market was valued at $1.69 billion in 2023 and is anticipated to grow to $3.67 billion by 2033. Factors such as enhanced healthcare policies and initiatives aimed at infection control significantly contribute to this expansion.Asia Pacific Sepsis Therapeutics Market Report:
In the Asia Pacific region, the Sepsis Therapeutics market was valued at $1.07 billion in 2023 and is expected to grow to approximately $2.32 billion by 2033, indicating significant market potential. Increasing healthcare expenditure and rising awareness of infectious diseases drive this growth.North America Sepsis Therapeutics Market Report:
The North American market for Sepsis Therapeutics was valued at $1.70 billion in 2023, with forecasts suggesting it will reach $3.68 billion by 2033. This is largely due to the presence of advanced healthcare systems, rising incidences of sepsis, and ongoing research.South America Sepsis Therapeutics Market Report:
The South American Sepsis Therapeutics market was valued at $0.16 billion in 2023 and is projected to reach $0.34 billion by 2033. The growth in this region is primarily attributed to improving healthcare infrastructure and increasing access to medical treatments.Middle East & Africa Sepsis Therapeutics Market Report:
The Middle East and Africa exhibited a market value of $0.68 billion in 2023, with expectations of growth to $1.48 billion by 2033. Increasing awareness and initiatives to combat healthcare-associated infections are pivotal to market growth in this region.Tell us your focus area and get a customized research report.
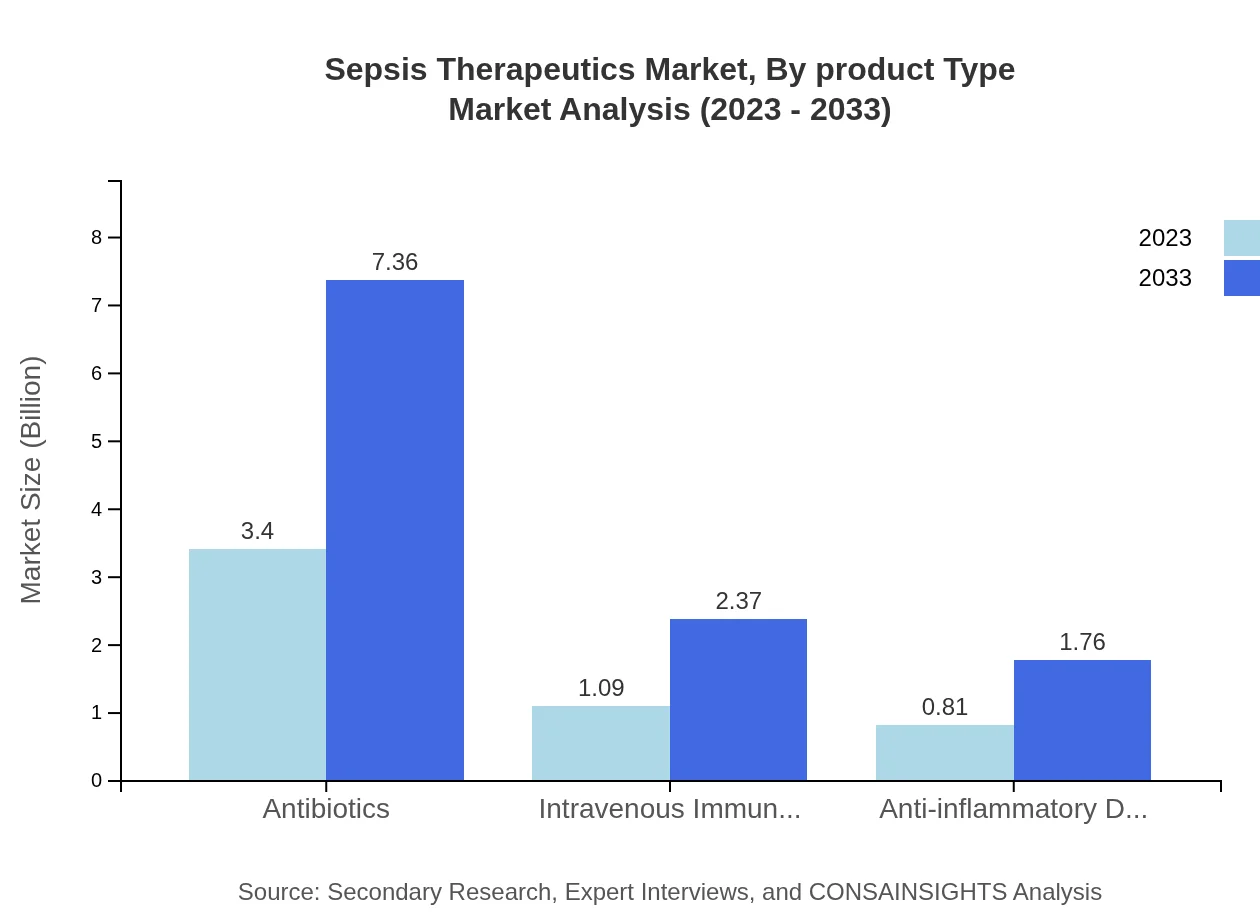
Sepsis Therapeutics Market Analysis By Product Type
The Sepsis Therapeutics market is robustly driven by various product types including Antibiotics, IVIG, and Anti-inflammatory drugs. Antibiotics are projected to maintain a significant market share, accounting for 64.09% in 2023, with expected growth to 64.09% in 2033. IVIG and Anti-inflammatory drugs also contribute substantially with 20.6% and 15.31% shares respectively throughout the forecasted period.
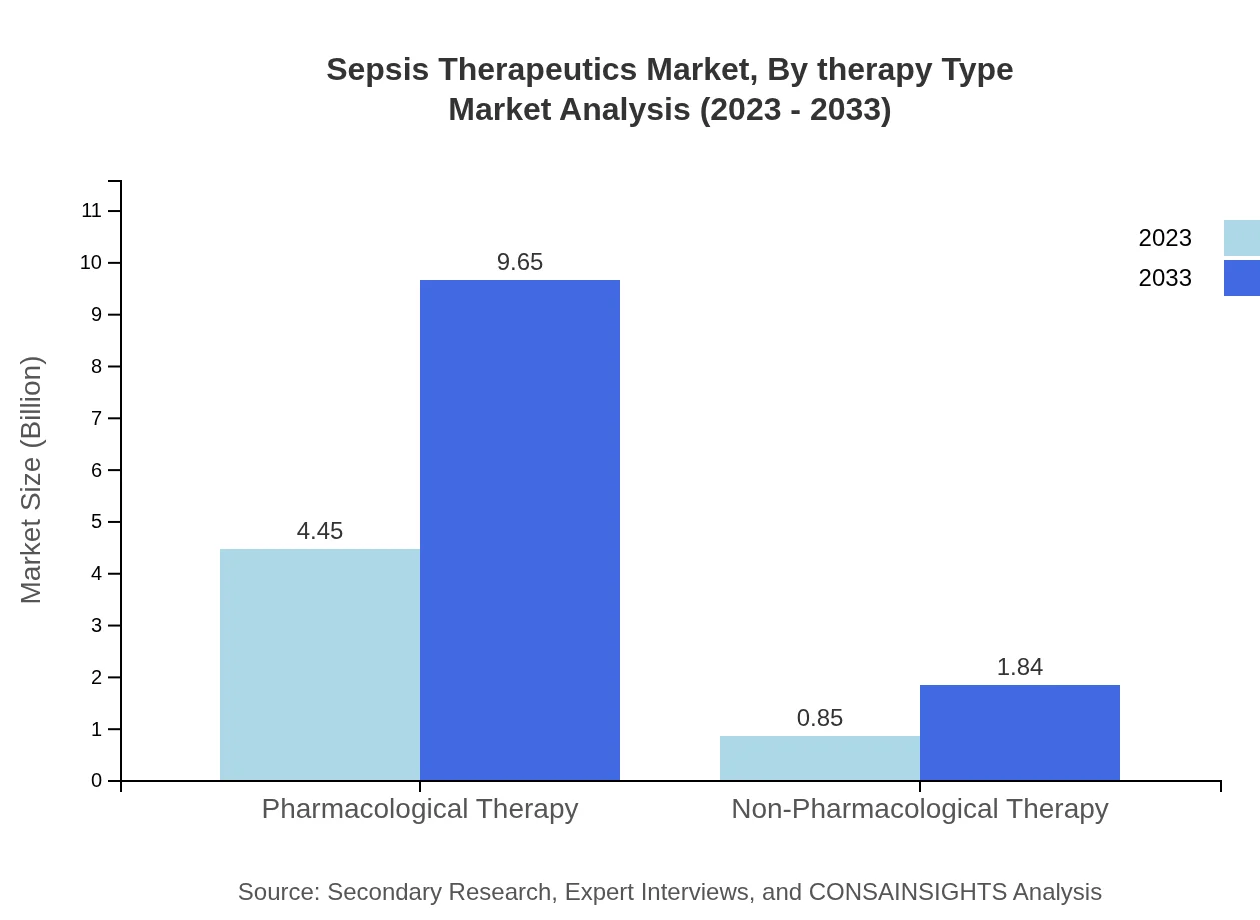
Sepsis Therapeutics Market Analysis By Therapy Type
Pharmacological therapy dominates the Sepsis Therapeutics market, holding a share of 83.96% in 2023 with consistent growth projected through to 2033. Non-pharmacological therapy comprises the remaining share, emphasizing a balanced approach in treatment methodologies.
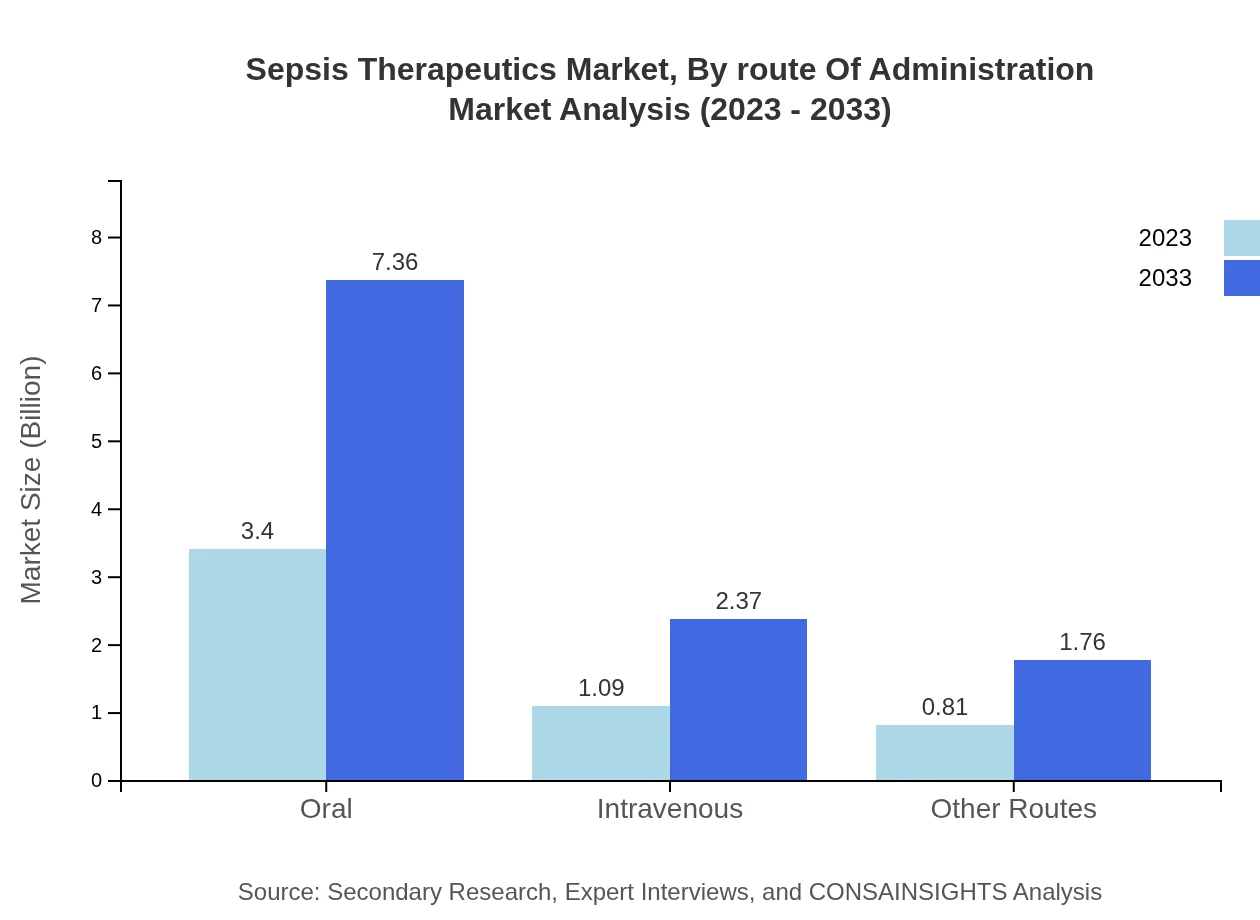
Sepsis Therapeutics Market Analysis By Route Of Administration
Oral administration retains a significant share of the market at 64.09% in 2023, reflecting ease of use and patient compliance. However, Intravenous routes also play an essential role, accounting for a growing share of 20.6% as many patients require urgent and effective treatment.
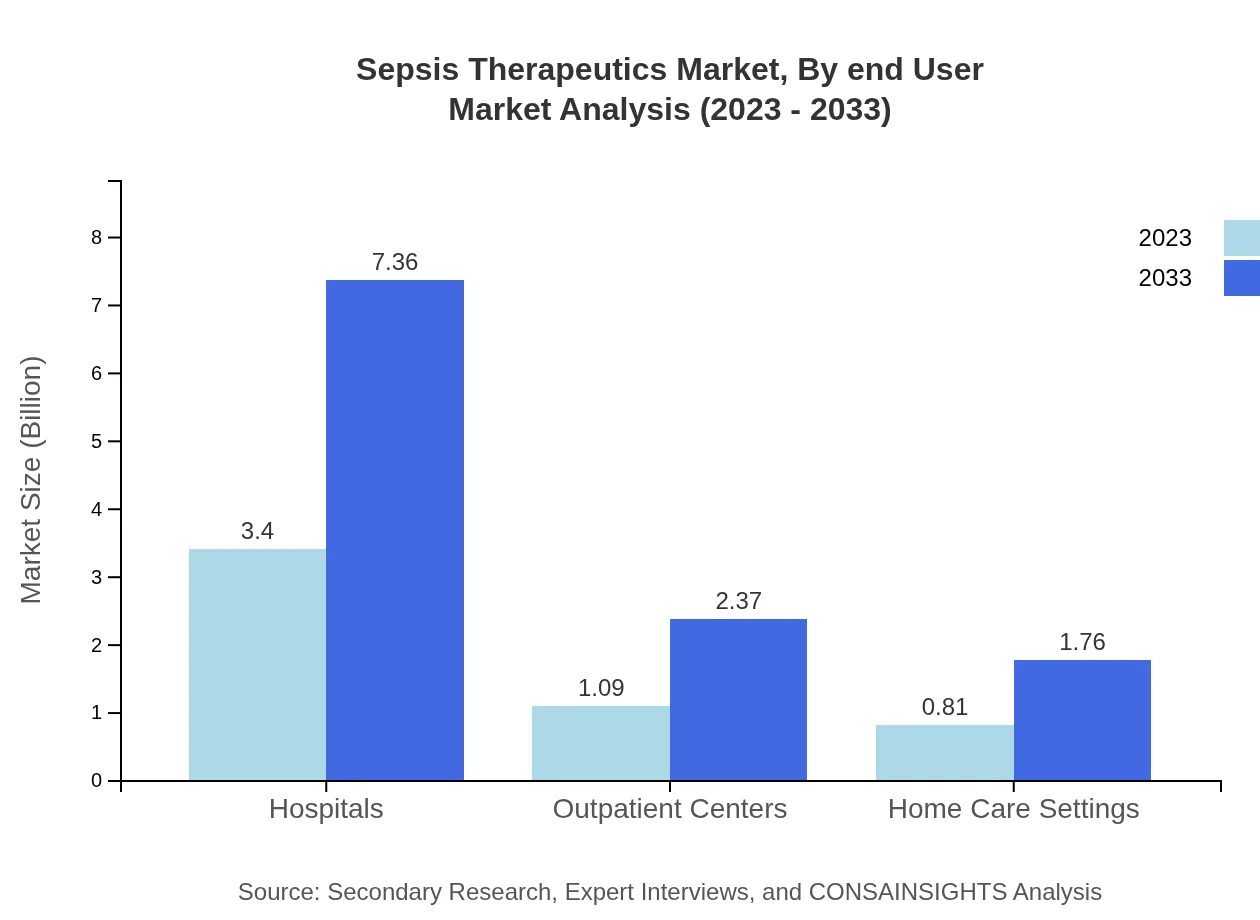
Sepsis Therapeutics Market Analysis By End User
Hospitals are the primary end-users of Sepsis Therapeutics, expected to maintain a dominant share of 64.09% in 2023. Outpatient centers are increasingly gaining attention, projected at 20.6%, alongside a growth in home care settings due to rising patient preference for home-based therapies.
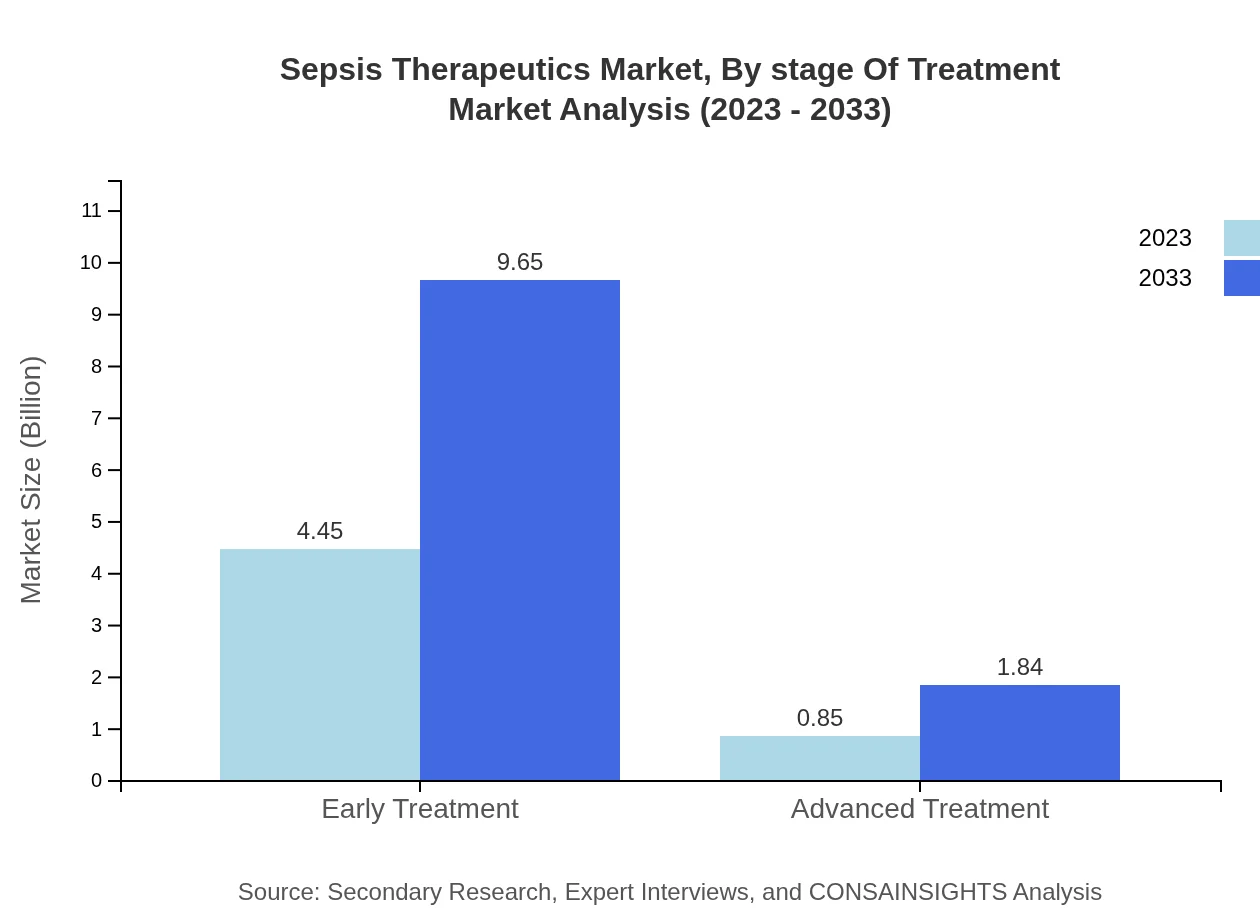
Sepsis Therapeutics Market Analysis By Stage Of Treatment
Early treatment remains pivotal in the management of sepsis, securing a share of 83.96% in 2023. Advanced treatment, though lesser in share at 16.04%, is essential for patients requiring further intervention post-initial therapy.
Sepsis Therapeutics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Sepsis Therapeutics Industry
Pfizer :
Pfizer is a leading pharmaceutical company extensively involved in the development of antibiotics and will continue to focus on sepsis treatment through innovative research and partnerships.Novo Nordisk:
Novo Nordisk specializes in treatments for various chronic diseases, with a growing interest in sepsis management, leveraging its expertise in infusion therapies.AstraZeneca:
AstraZeneca is known for its biologics portfolio aimed at severe infections, continually advancing research projects focused on sepsis therapeutics.Merck & Co.:
Merck & Co. has a strong presence in infectious diseases and is actively engaged in developing novel therapies aimed at combating sepsis.GlaxoSmithKline:
GlaxoSmithKline is pioneering vaccine development alongside therapeutic solutions for sepsis, positioning itself as a significant player in the infection prevention domain.We're grateful to work with incredible clients.









FAQs
What is the market size of sepsis Therapeutics?
The sepsis-therapeutics market is projected to reach $5.3 billion by 2033, growing at a CAGR of 7.8% from its current valuation. This growth reflects the increasing emphasis on improving sepsis management and treatment protocols globally.
What are the key market players or companies in this sepsis Therapeutics industry?
Key players in the sepsis-therapeutics market include pharmaceutical companies focusing on antibiotic treatments, immunotherapies, and biotechnology firms that develop innovative therapies targeting sepsis management. The competitive landscape is characterized by both established enterprises and new entrants.
What are the primary factors driving the growth in the sepsis Therapeutics industry?
Growth in the sepsis-therapeutics market is driven by rising sepsis incidence rates, increased awareness, advancements in diagnostics and treatment options, and stringent healthcare regulations mandating better sepsis management. Additionally, investments in research and development further boost market progress.
Which region is the fastest Growing in the sepsis Therapeutics?
The fastest-growing region for sepsis-therapeutics is projected to be Europe, with market growth from $1.69 billion in 2023 to $3.67 billion by 2033. This growth is fueled by enhanced healthcare infrastructure and a growing focus on infectious disease management.
Does ConsaInsights provide customized market report data for the sepsis Therapeutics industry?
Yes, ConsaInsights offers customized market report data tailored to specific research needs in the sepsis-therapeutics industry. Clients can request specific insights based on regional, segment, and trend analysis.
What deliverables can I expect from this sepsis Therapeutics market research project?
Deliverables from this market research project include a detailed report comprising market size analysis, CAGR forecasts, insight into key players, regional breakdowns, segment analysis, and trend observations essential for strategic decision-making.
What are the market trends of sepsis Therapeutics?
Current market trends in sepsis-therapeutics include a significant shift towards early treatment protocols, increased adoption of pharmacological interventions, and integration of advanced diagnostic tools, alongside a focus on personalized medicine to enhance patient outcomes.