Serological Transplant Diagnostics Market Report
Published Date: 31 January 2026 | Report Code: serological-transplant-diagnostics
Serological Transplant Diagnostics Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Serological Transplant Diagnostics market, examining key insights, market size, trends, and forecasts for the period from 2023 to 2033. Data-driven insights into regional performance and industry dynamics are encompassed.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
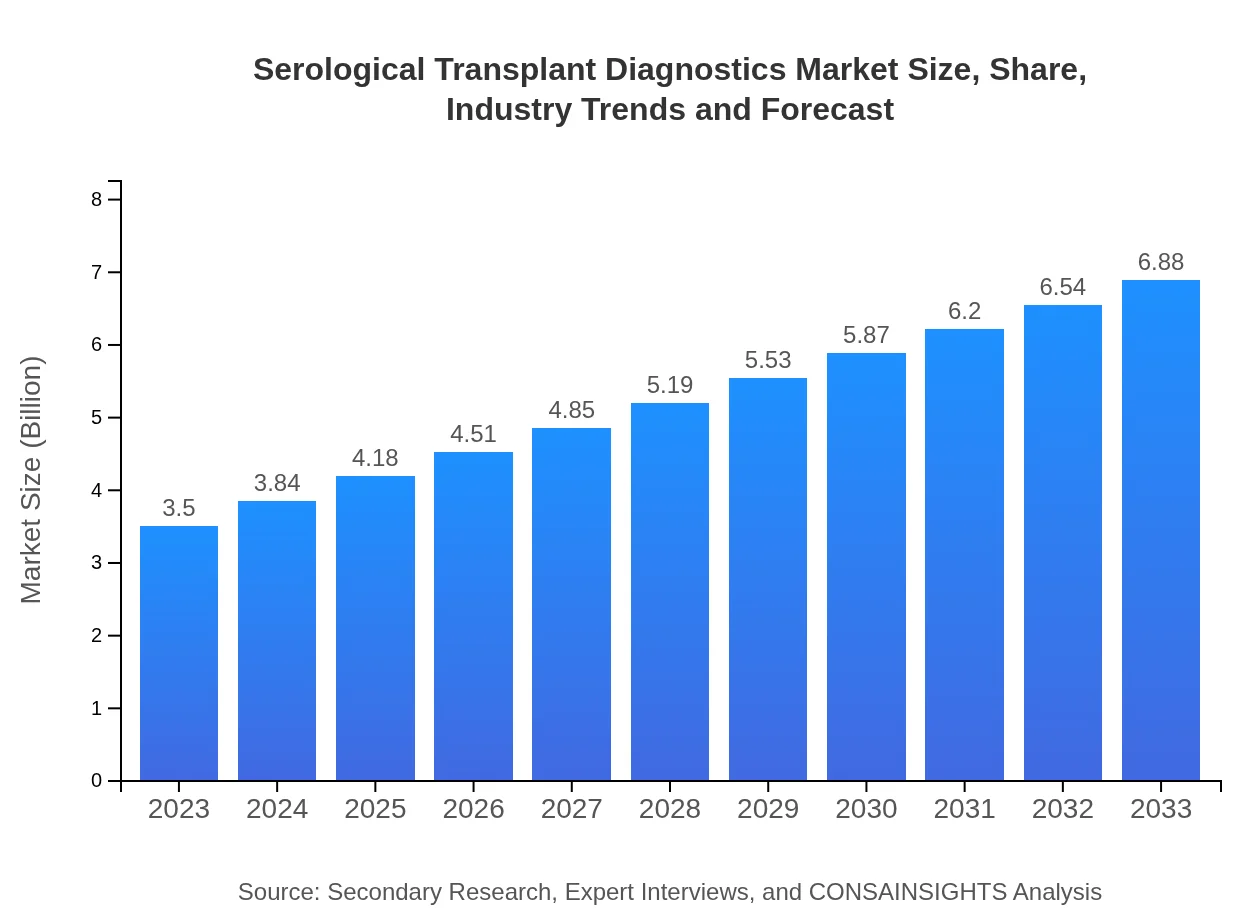
| 2023 Market Size | $3.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $6.88 Billion |
| Top Companies | Abbott Laboratories, Thermo Fisher Scientific Inc., Roche Diagnostics |
| Last Modified Date | 31 January 2026 |
Serological Transplant Diagnostics Market Overview
Customize Serological Transplant Diagnostics Market Report market research report
- ✔ Get in-depth analysis of Serological Transplant Diagnostics market size, growth, and forecasts.
- ✔ Understand Serological Transplant Diagnostics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Serological Transplant Diagnostics
What is the Market Size & CAGR of Serological Transplant Diagnostics market in 2023?
Serological Transplant Diagnostics Industry Analysis
Serological Transplant Diagnostics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Serological Transplant Diagnostics Market Analysis Report by Region
Europe Serological Transplant Diagnostics Market Report:
The European Serological Transplant Diagnostics market is forecasted to increase from $1.13 billion in 2023 to $2.22 billion in 2033. Stringent regulatory frameworks and advanced healthcare technologies promote significant advancements in diagnostic methodologies.Asia Pacific Serological Transplant Diagnostics Market Report:
In Asia-Pacific, the Serological Transplant Diagnostics market is projected to grow from $0.69 billion in 2023 to $1.35 billion in 2033, driven by increasing organ transplant cases, rising healthcare infrastructure investments, and a growing population's healthcare awareness.North America Serological Transplant Diagnostics Market Report:
North America remains the largest market, expected to grow from $1.14 billion in 2023 to $2.23 billion by 2033. Increased organ transplant rates, advanced healthcare systems, and a high prevalence of chronic diseases significantly drive this growth.South America Serological Transplant Diagnostics Market Report:
The South American market shows modest growth, increasing from $0.07 billion in 2023 to $0.14 billion by 2033 due to limited healthcare spending but is poised for gradual growth with improved medical access and rising health awareness.Middle East & Africa Serological Transplant Diagnostics Market Report:
The Middle East and Africa region's market is anticipated to grow from $0.48 billion in 2023 to $0.94 billion by 2033. Factors such as improving healthcare facilities, rising organ donation rates, and increasing awareness support the growth of the market.Tell us your focus area and get a customized research report.
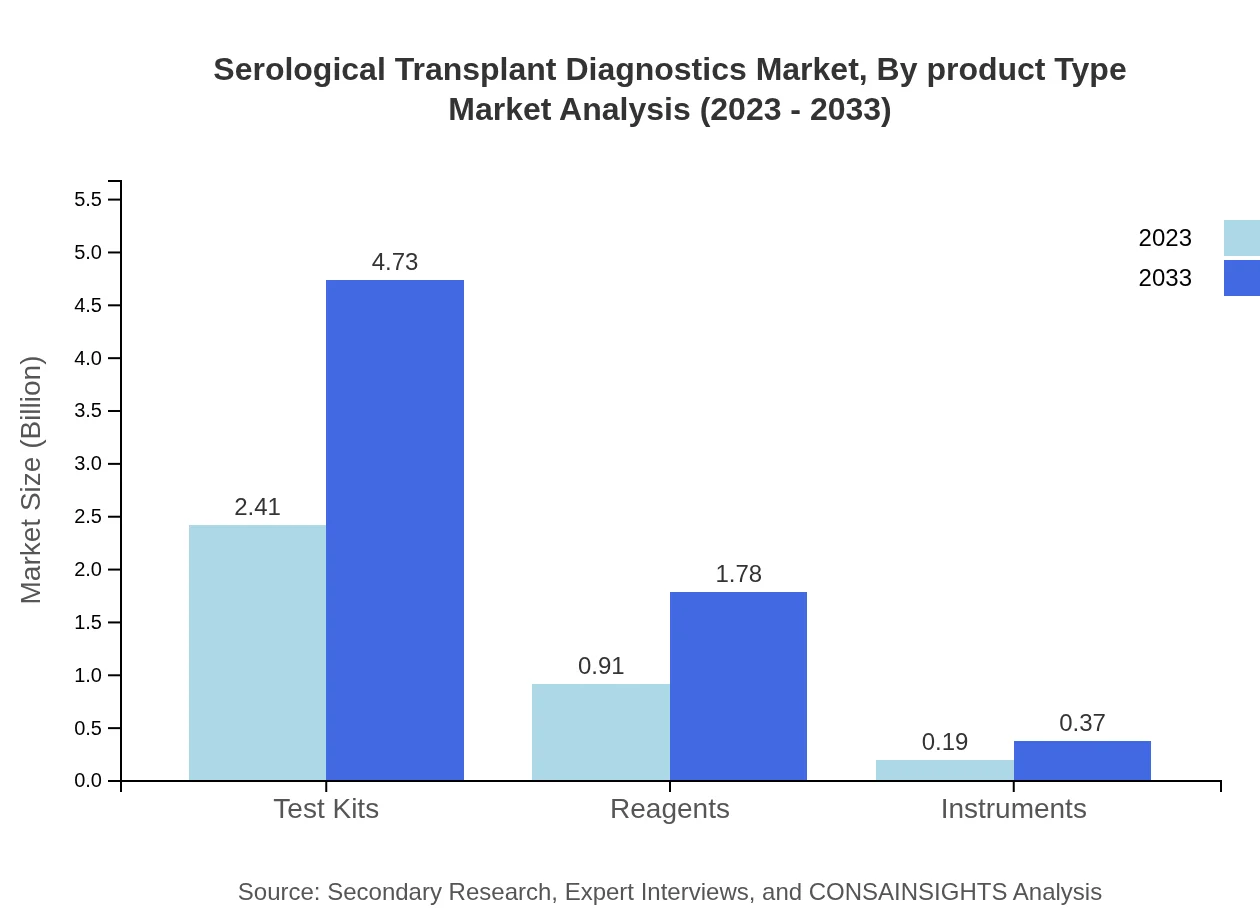
Serological Transplant Diagnostics Market Analysis By Product Type
The product type segment showcases test kits leading the market with a size of $2.41 billion in 2023, expected to rise to $4.73 billion by 2033, encapsulating 68.72% market share. Reagents hold the second-largest share at $0.91 billion, highlighting their importance in test reliability and detection efficiency, while instruments account for $0.19 billion.
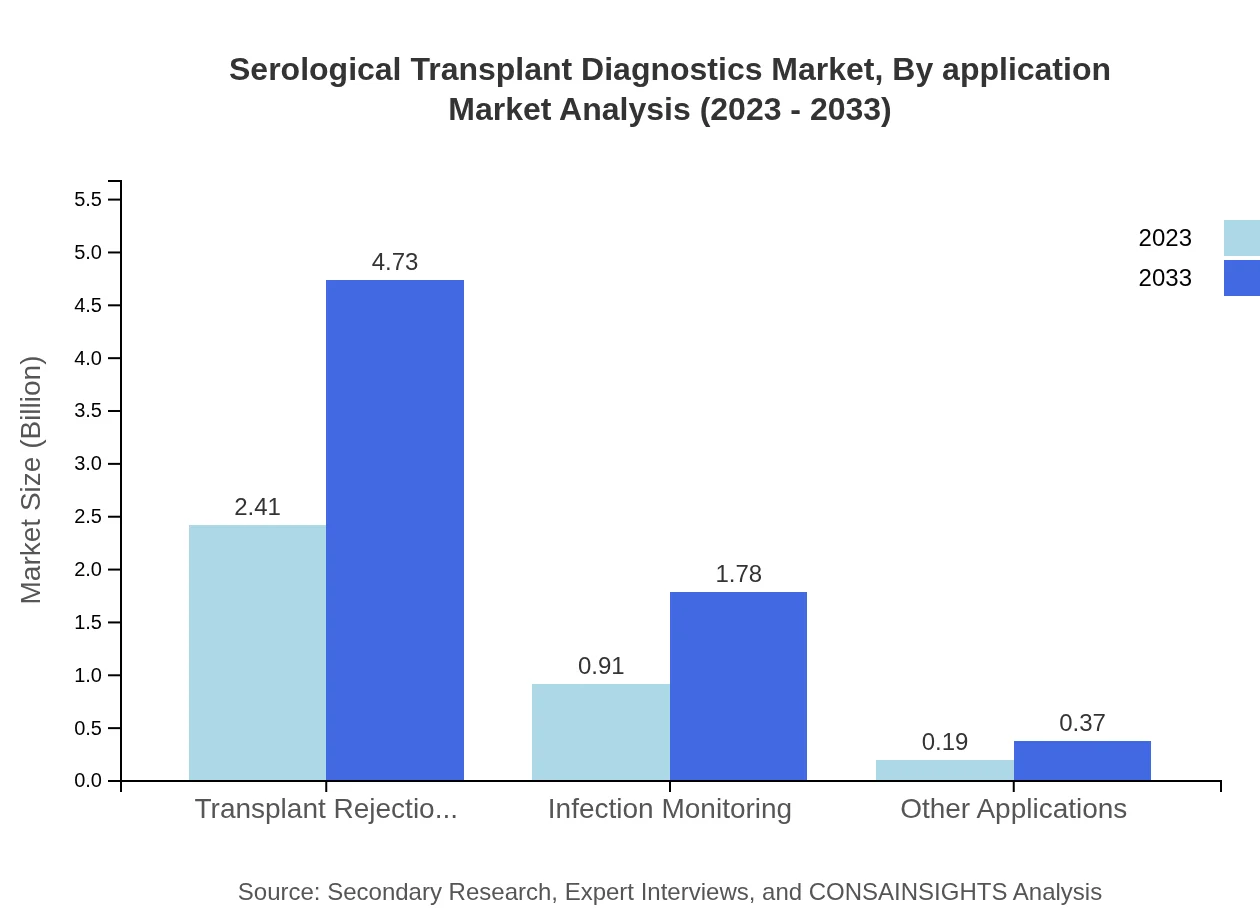
Serological Transplant Diagnostics Market Analysis By Application
Transplant rejection diagnostics dominate this segment, valued at $2.41 billion in 2023, with a market share of 68.72%. Infection monitoring follows, valued at $0.91 billion, signifying the critical need for infection control post-transplantation, while other applications capture $0.19 billion.
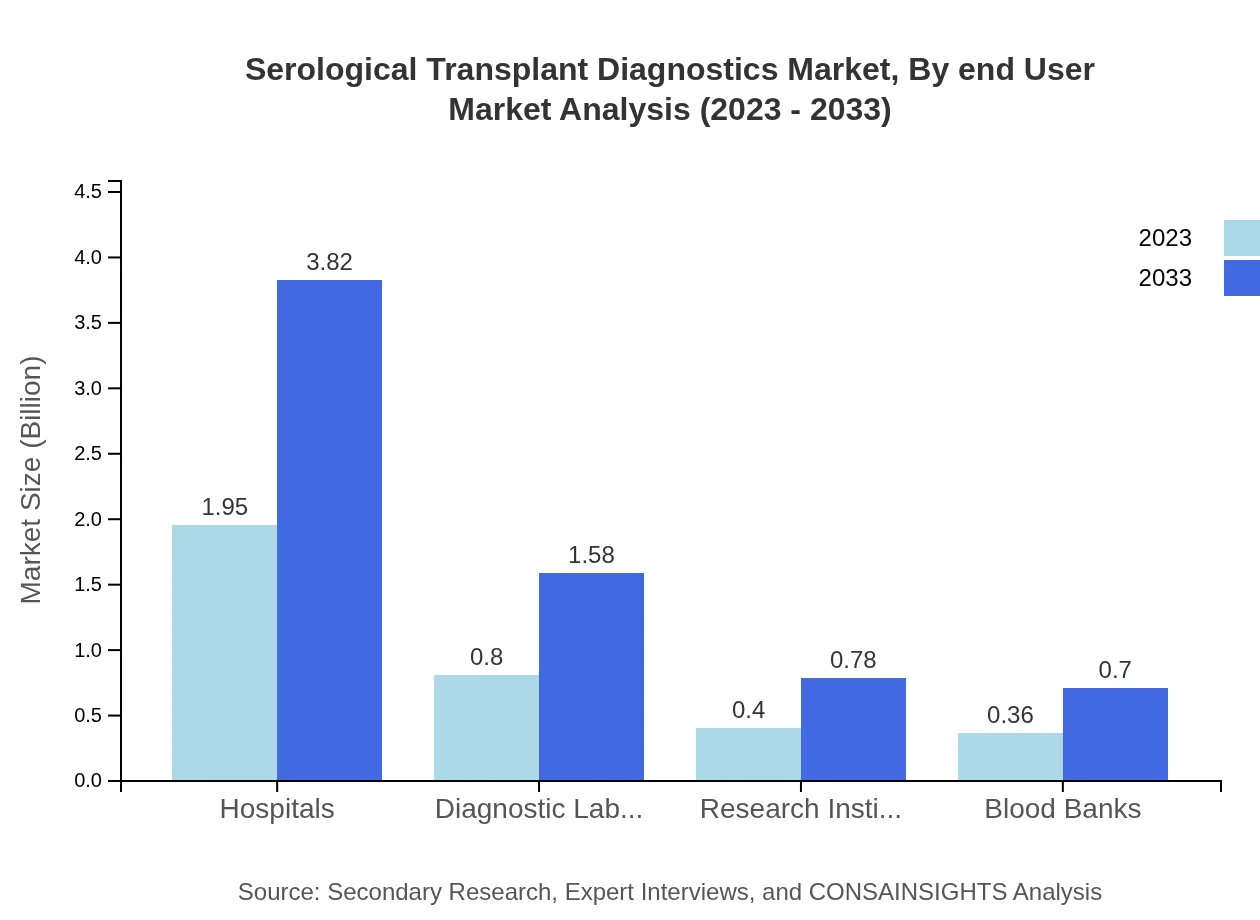
Serological Transplant Diagnostics Market Analysis By End User
Hospitals are the major end-users, with a market share of 55.58% valued at $1.95 billion in 2023 and expected to expand to $3.82 billion by 2033. Diagnostic laboratories follow with significant contributions valued at $0.80 billion, representing 22.95% of the market.
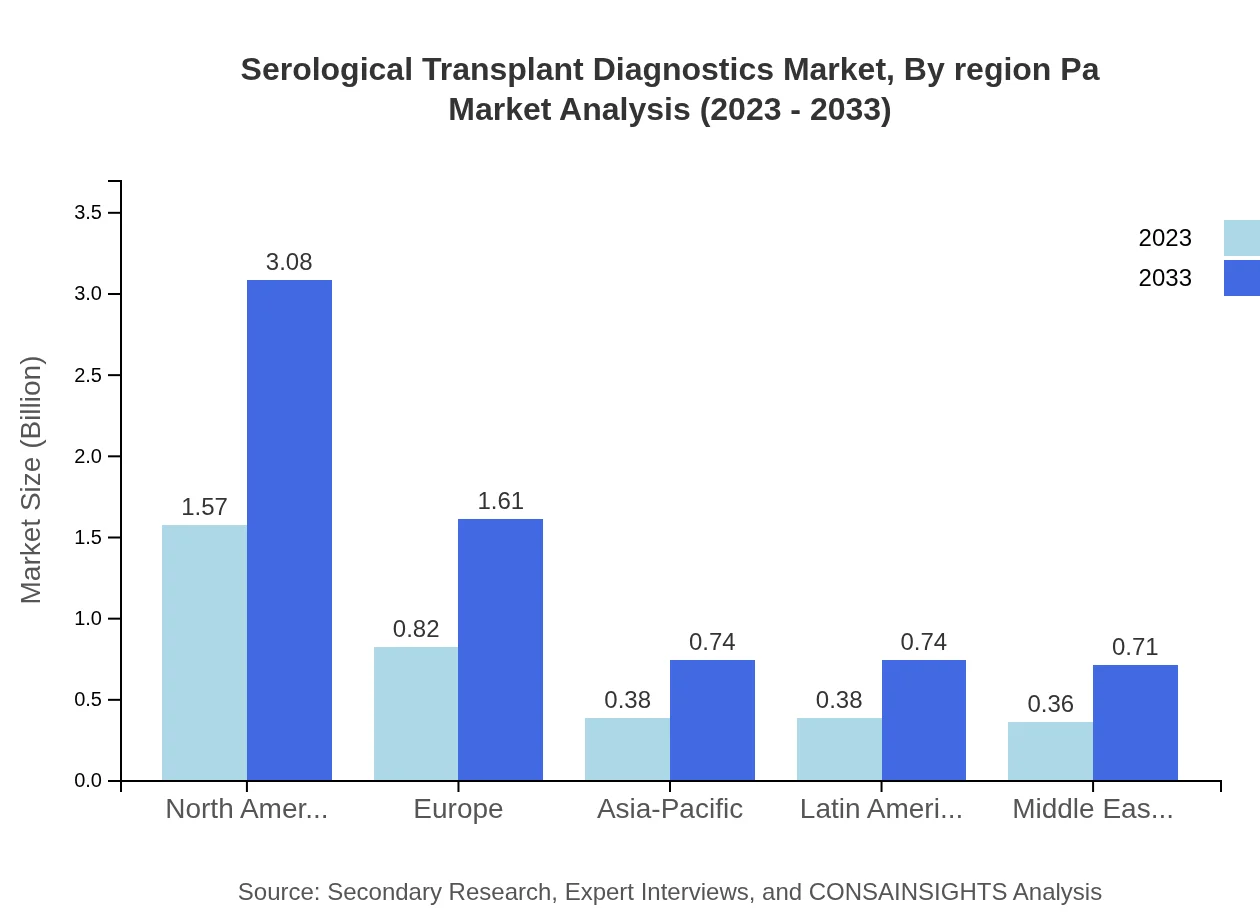
Serological Transplant Diagnostics Market Analysis By Region Pa
North America remains at the forefront of the regional breakdown, showcasing a size of $1.57 billion in 2023 while maintaining a considerable market share of 44.79%. Europe and Asia Pacific also display healthy growth patterns, echoing the increasing demands and awareness surrounding transplant diagnostics.
Serological Transplant Diagnostics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Serological Transplant Diagnostics Industry
Abbott Laboratories:
A global leader in medical diagnostics, Abbott excels in developing innovative tools to monitor and diagnose various health conditions, including transplant diagnostics.Thermo Fisher Scientific Inc.:
Known for its advanced technological solutions, Thermo Fisher specializes in accurate and efficient diagnostic systems, supporting the serological transplant diagnostics sector sector.Roche Diagnostics:
Roche is a pioneer in the field, providing a wide array of diagnostics tools that enhance the quality and efficiency of transplant monitoring.We're grateful to work with incredible clients.









FAQs
What is the market size of serological Transplant Diagnostics?
The serological transplant diagnostics market is valued at approximately $3.5 billion and is projected to grow at a CAGR of 6.8% from 2023 to 2033. This growth reflects the increasing demand for effective transplant rejection testing and infection monitoring.
What are the key market players or companies in this serological Transplant Diagnostics industry?
Key players in the serological transplant diagnostics market include large diagnostic companies, hospitals, and research institutes. These entities are pivotal in enhancing diagnostic accuracy, offering advanced testing solutions, and contributing to market expansion through innovative technology.
What are the primary factors driving the growth in the serological transplant diagnostics industry?
The growth in the serological transplant diagnostics industry is driven by increasing transplant surgeries, rising awareness about transplant rejection, advancements in diagnostic technology, and the growing need for efficient infection monitoring post-transplant to ensure patient safety.
Which region is the fastest Growing in the serological transplant diagnostics?
Asia-Pacific is emerging as the fastest-growing region in the serological transplant diagnostics market, with its market size expected to grow from $0.69 billion in 2023 to $1.35 billion in 2033, representing significant development in healthcare infrastructure and diagnostic capabilities.
Does ConsaInsights provide customized market report data for the serological transplant diagnostics industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the serological transplant diagnostics industry. This includes detailed analyses and forecasts based on unique client specifications, ensuring relevance and actionable insights.
What deliverables can I expect from this serological transplant diagnostics market research project?
Deliverables from the serological transplant diagnostics market research project typically include a comprehensive report with market size analysis, growth forecasts, competitive landscape, regional insights, and segmentation data, along with actionable recommendations for stakeholders.
What are the market trends of serological transplant diagnostics?
Current market trends in serological transplant diagnostics include a shift towards molecular diagnostics, increasing demand for rapid test kits, advancements in automation technology in laboratories, and a focus on personalized medicine tailored to individual transplant recipients.