Single Use Medical Device Reprocessing Market Report
Published Date: 31 January 2026 | Report Code: single-use-medical-device-reprocessing
Single Use Medical Device Reprocessing Market Size, Share, Industry Trends and Forecast to 2033
This report provides a detailed analysis of the Single Use Medical Device Reprocessing market, highlighting market trends, segmentation, industry analysis, and forecasts from 2023 to 2033. Key insights on regional performance and leading market players are also included.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
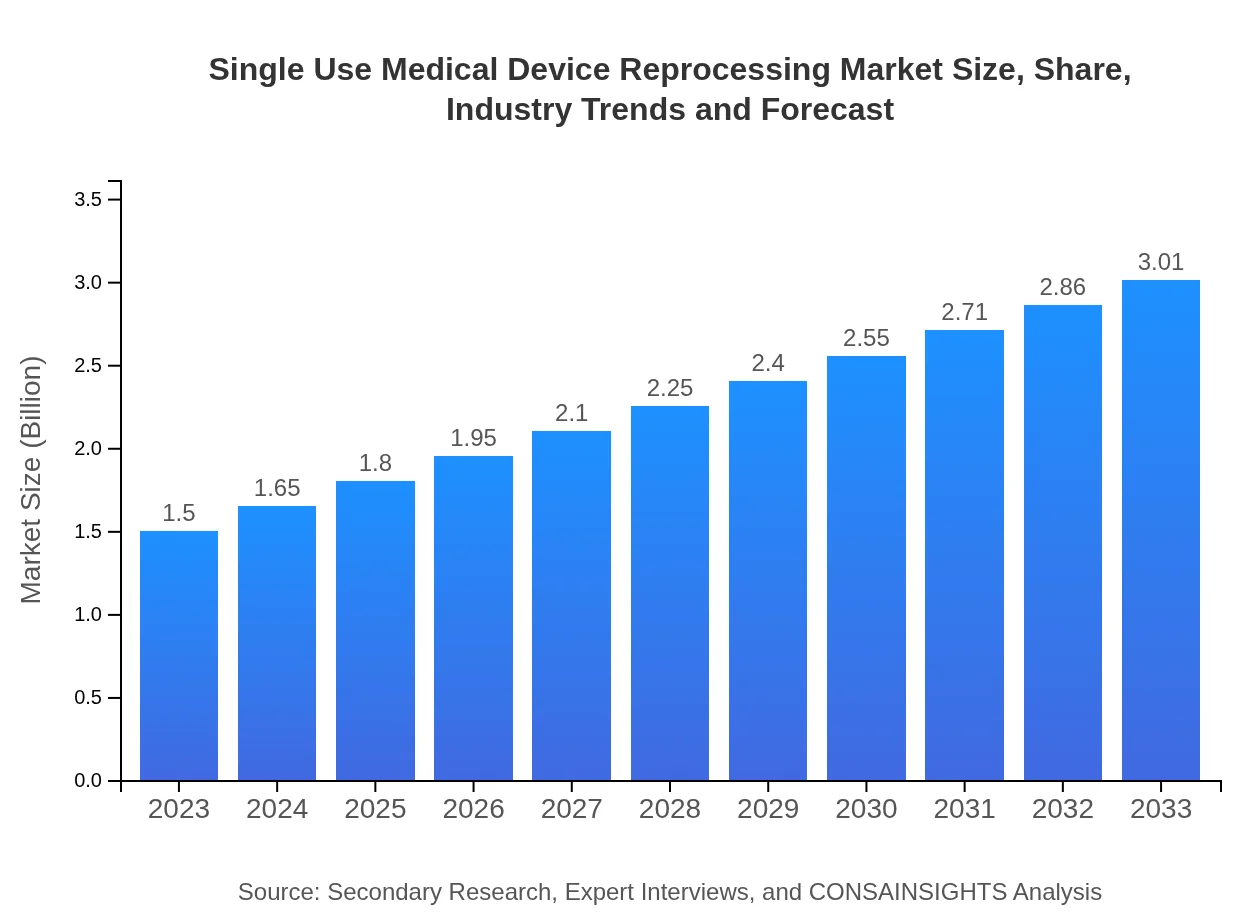
| 2023 Market Size | $1.50 Billion |
| CAGR (2023-2033) | 7.0% |
| 2033 Market Size | $3.01 Billion |
| Top Companies | Stericycle, Medline Industries, Inc., Vanguard Ag, Inc. |
| Last Modified Date | 31 January 2026 |
Single Use Medical Device Reprocessing Market Overview
Customize Single Use Medical Device Reprocessing Market Report market research report
- ✔ Get in-depth analysis of Single Use Medical Device Reprocessing market size, growth, and forecasts.
- ✔ Understand Single Use Medical Device Reprocessing's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Single Use Medical Device Reprocessing
What is the Market Size & CAGR of Single Use Medical Device Reprocessing market in 2023?
Single Use Medical Device Reprocessing Industry Analysis
Single Use Medical Device Reprocessing Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Single Use Medical Device Reprocessing Market Analysis Report by Region
Europe Single Use Medical Device Reprocessing Market Report:
The European market for SUMDR is projected to grow from $0.44 billion in 2023 to $0.88 billion in 2033. Strong regulations favoring environmental sustainability, combined with an increasing number of hospitals adopting reprocessing methods, are driving this growth.Asia Pacific Single Use Medical Device Reprocessing Market Report:
The Asia Pacific is expected to witness substantial growth in the SUMDR market, projected to reach $0.60 billion by 2033 from $0.30 billion in 2023. Key factors include growing healthcare expenditure and an increase in awareness about the benefits of medical waste management.North America Single Use Medical Device Reprocessing Market Report:
North America holds the largest share of the SUMDR market, with an estimated market size increasing from $0.52 billion in 2023 to $1.05 billion by 2033. This growth is primarily driven by the high prevalence of surgical procedures, stringent regulatory frameworks supporting SUMDR implementation, and a strong push toward sustainability in healthcare.South America Single Use Medical Device Reprocessing Market Report:
In South America, the SUMDR market is anticipated to grow from $0.07 billion in 2023 to $0.15 billion in 2033. The market is driven by increasing hospital activities and the necessity for cost-effective healthcare solutions in a region faced with economic challenges.Middle East & Africa Single Use Medical Device Reprocessing Market Report:
The Middle East and Africa market is expected to grow from $0.17 billion in 2023 to $0.34 billion by 2033. Factors contributing to this growth include increased public healthcare spending and enhancements in healthcare infrastructure, especially within urban areas.Tell us your focus area and get a customized research report.
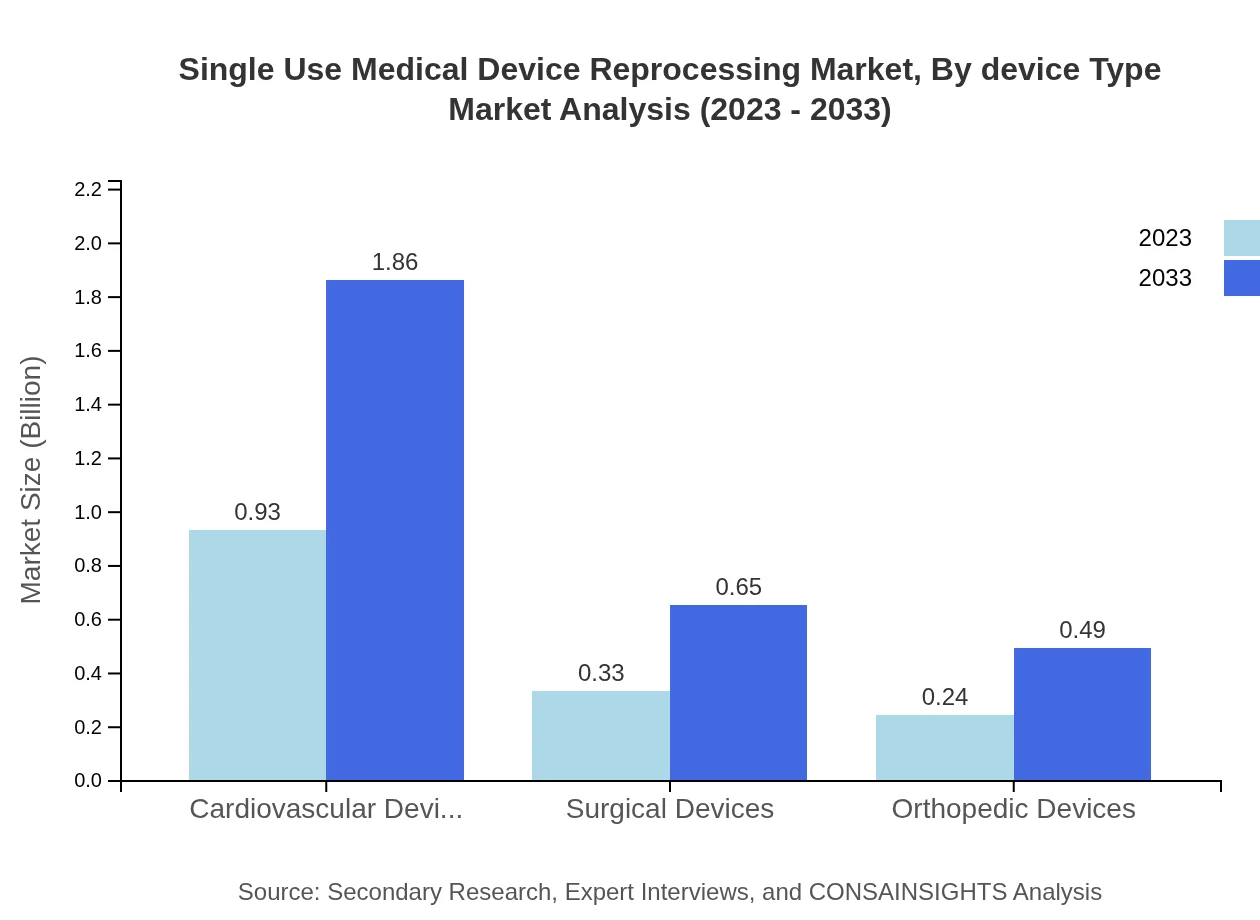
Single Use Medical Device Reprocessing Market Analysis By Device Type
The market size for cardiovascular devices is expected to double by 2033, rising from $0.93 billion to $1.86 billion. Surgical devices and orthopedic devices will also see significant growth, with surgical device market size rising from $0.33 billion to $0.65 billion, and orthopedic devices from $0.24 billion to $0.49 billion, indicating the critical nature of these devices in reprocessing initiatives.
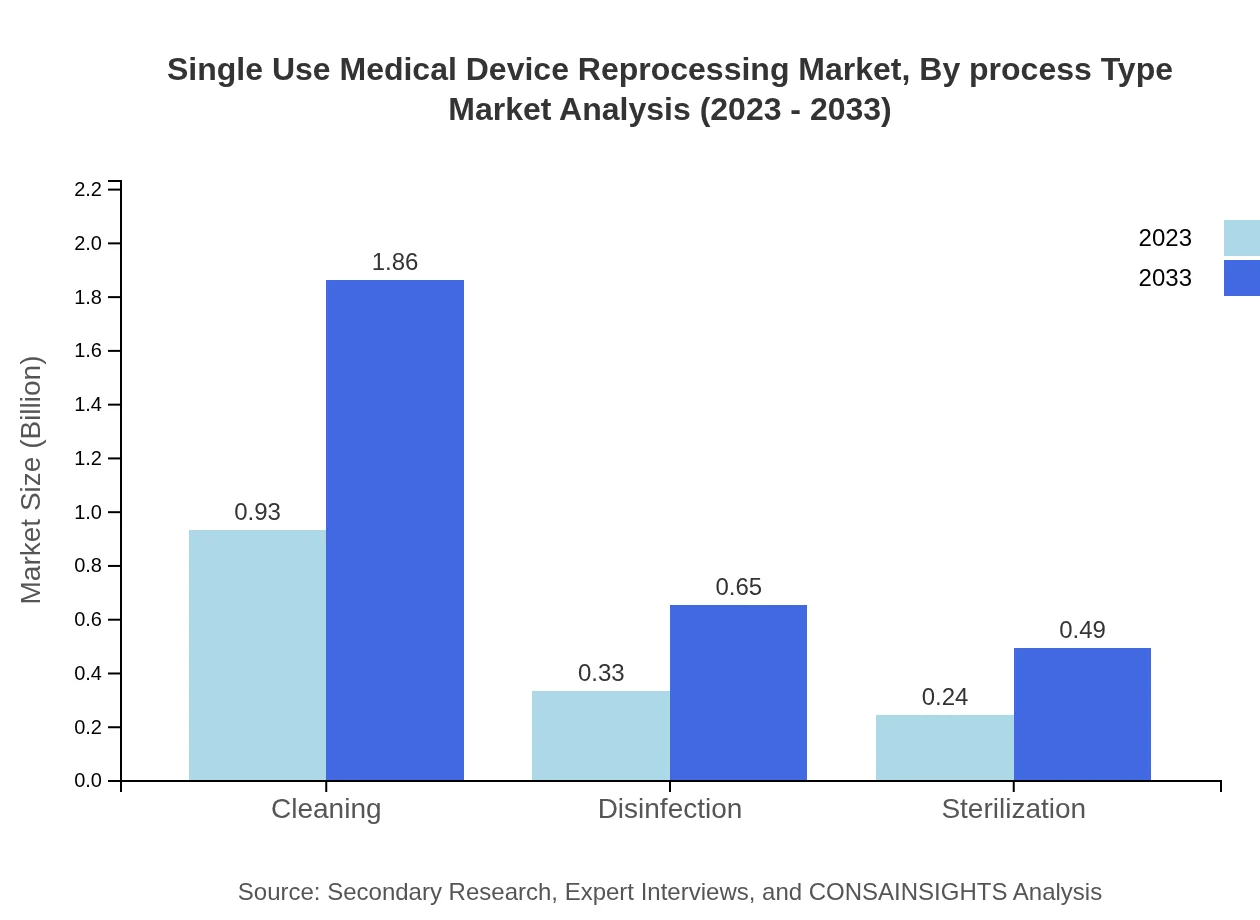
Single Use Medical Device Reprocessing Market Analysis By Process Type
Among various process types, cleaning services are anticipated to dominate the market. This segment is projected to expand from $0.93 billion in 2023 to $1.86 billion by 2033. Disinfection and sterilization processes will also grow, increasing from $0.33 billion to $0.65 billion and $0.24 billion to $0.49 billion, respectively.
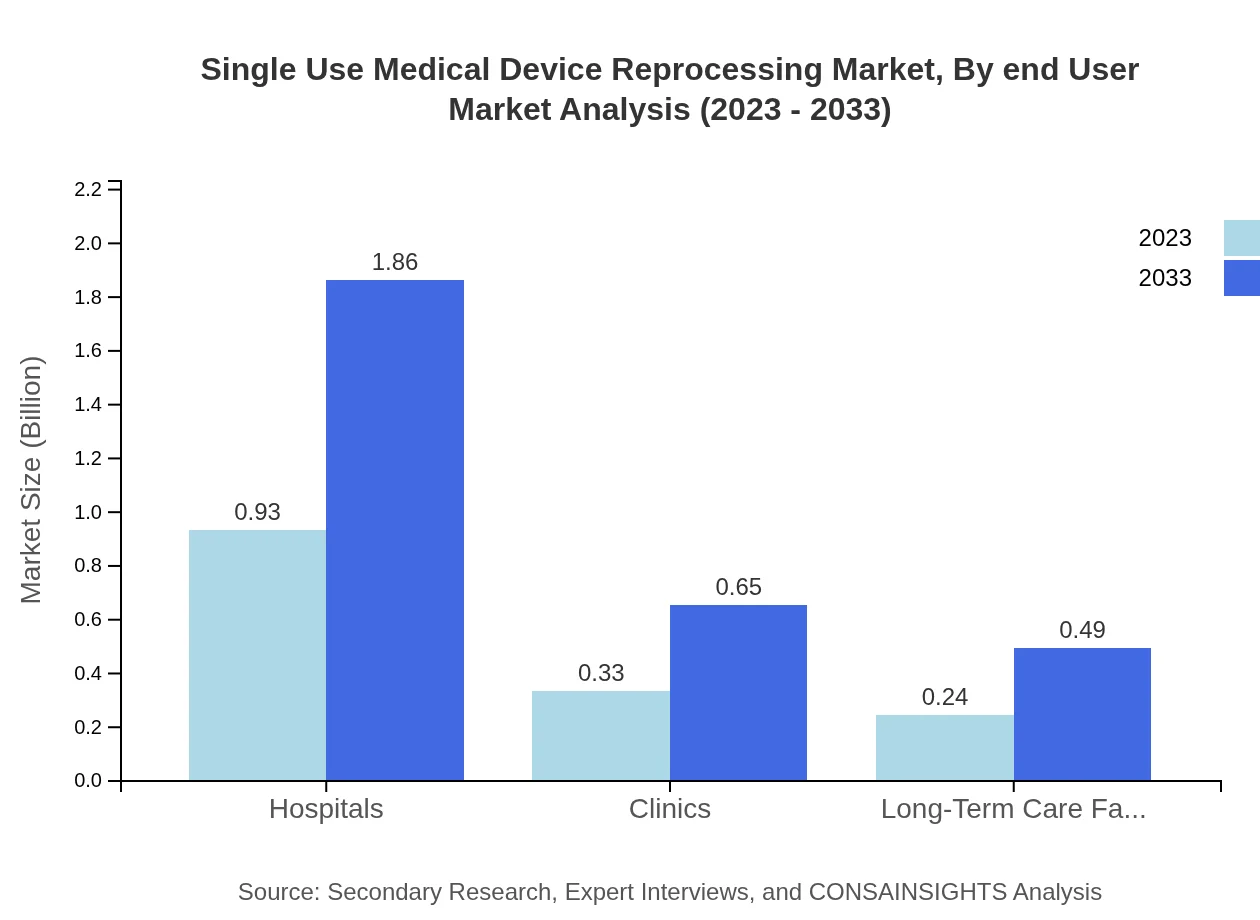
Single Use Medical Device Reprocessing Market Analysis By End User
Hospitals and health systems dominate the market, expected to grow from $1.24 billion in 2023 to $2.49 billion by 2033. Clinics and long-term care facilities are also expanding, demonstrating a growing trend toward reprocessing among various healthcare providers.
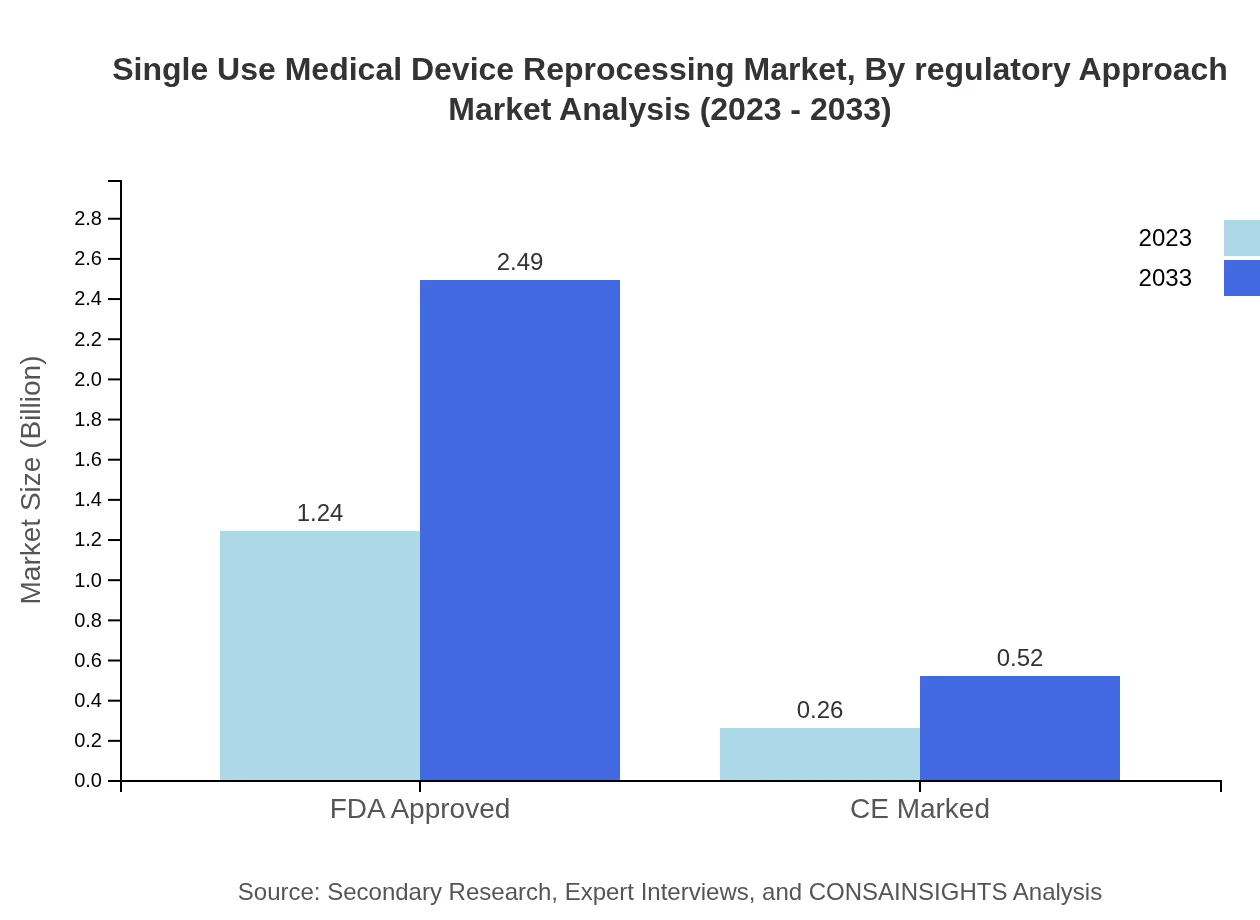
Single Use Medical Device Reprocessing Market Analysis By Regulatory Approach
The FDA-approved devices will continue to dominate the market, projected to maintain an 82.84% market share throughout the forecast period. CE marked devices will also hold a notable share, expected to account for 17.16%.
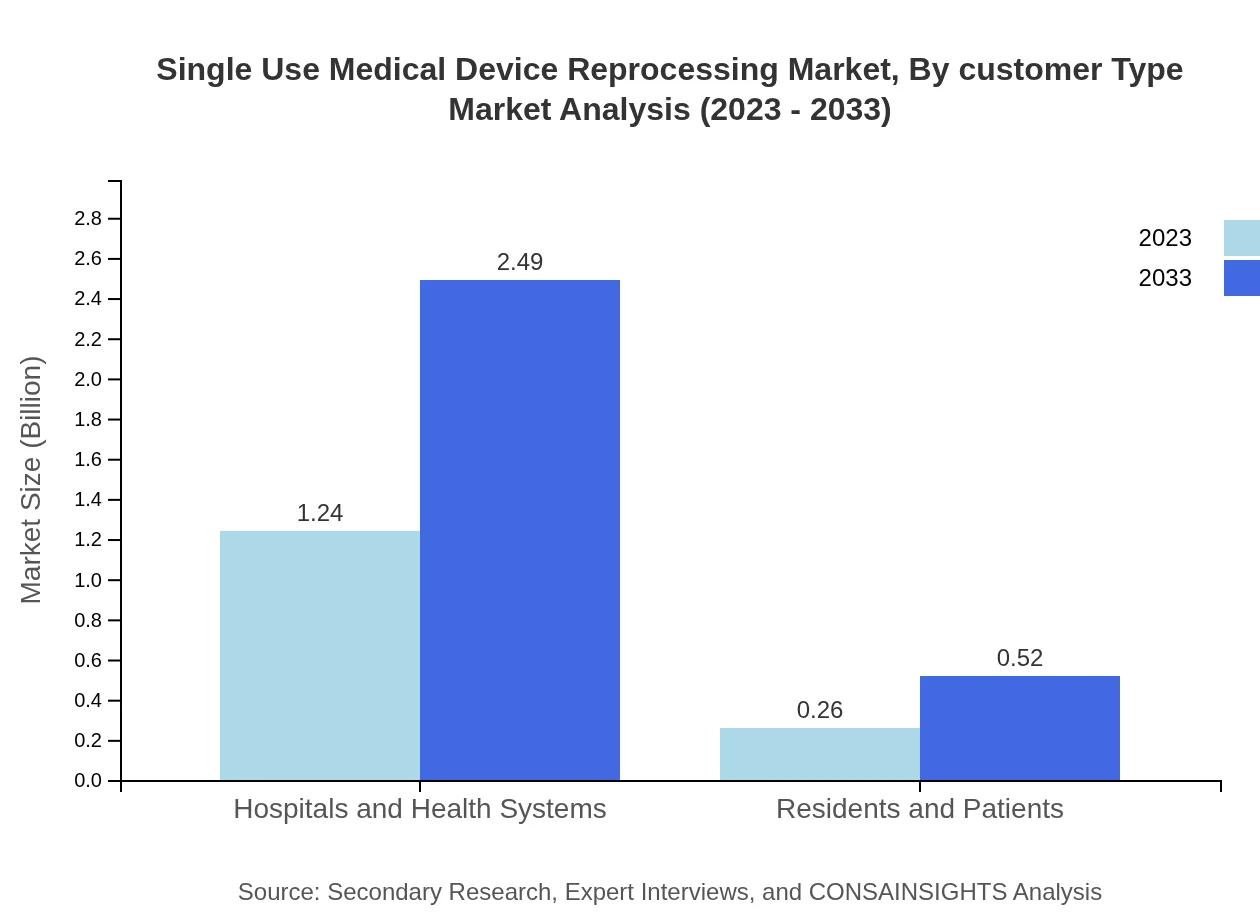
Single Use Medical Device Reprocessing Market Analysis By Customer Type
The market is primarily driven by hospitals, which are expected to account for a significant share as they increasingly adopt reprocessing practices to manage costs effectively while ensuring patient safety.
Single Use Medical Device Reprocessing Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Single Use Medical Device Reprocessing Industry
Stericycle:
Stericycle is a leading provider of medical waste management services, actively involved in the reprocessing of single-use medical devices, providing sustainable and compliant solutions across various healthcare settings.Medline Industries, Inc.:
Medline is a global manufacturer and distributor of medical supplies, heavily invested in developing innovative reprocessing programs that enhance the sustainability of healthcare operations.Vanguard Ag, Inc.:
Vanguard Ag specializes in reprocessing medical devices, particularly in surgical environments, promoting eco-friendly practices while maintaining high safety standards.We're grateful to work with incredible clients.









FAQs
What is the market size of single Use Medical Device Reprocessing?
The global single-use medical device reprocessing market is currently valued at $1.5 billion, with a projected CAGR of 7.0% from 2023 to 2033. This growth reflects increasing demand for sustainable healthcare practices and cost-efficient medical solutions.
What are the key market players or companies in this single Use Medical Device Reprocessing industry?
Key players in the single-use medical device reprocessing industry include Medline Industries, ReNu Medical, and Sterilmed. These companies are recognized for their innovation in technology and commitment to enhancing the efficiency of medical waste management.
What are the primary factors driving the growth in the single Use Medical Device Reprocessing industry?
The growth in the single-use medical device reprocessing industry is driven by rising healthcare costs, increasing focus on sustainability, regulatory support, and advancements in reprocessing technologies that enhance safety and effectiveness of recycled devices.
Which region is the fastest Growing in the single Use Medical Device Reprocessing?
North America is currently the fastest-growing region in the single-use medical device reprocessing market, expected to increase from $0.52 billion in 2023 to $1.05 billion in 2033, highlighting a strong focus on health sustainability.
Does ConsaInsights provide customized market report data for the single Use Medical Device Reprocessing industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the single-use medical device reprocessing industry, ensuring comprehensive insights that align with client objectives and regional market conditions.
What deliverables can I expect from this single Use Medical Device Reprocessing market research project?
Deliverables from the single-use medical device reprocessing market research project include detailed market analysis, growth forecasts, competitive landscapes, consumer insights, and strategic recommendations based on thorough data interpretation and analysis.
What are the market trends of single Use Medical Device Reprocessing?
Current trends in the single-use medical device reprocessing market include increased adoption of technology for cleaning and sterilization processes, growing partnerships between hospitals and reprocessing companies, and expanding regulations favoring eco-friendly medical practices.