Skin Perfusion Pressure Testing Devices Market Report
Published Date: 31 January 2026 | Report Code: skin-perfusion-pressure-testing-devices
Skin Perfusion Pressure Testing Devices Market Size, Share, Industry Trends and Forecast to 2033
This report delves into the Skin Perfusion Pressure Testing Devices market, offering insights on market trends, developments, and forecasts from 2023 to 2033. It highlights market size, growth rates, regional analyses, and technology advancements.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
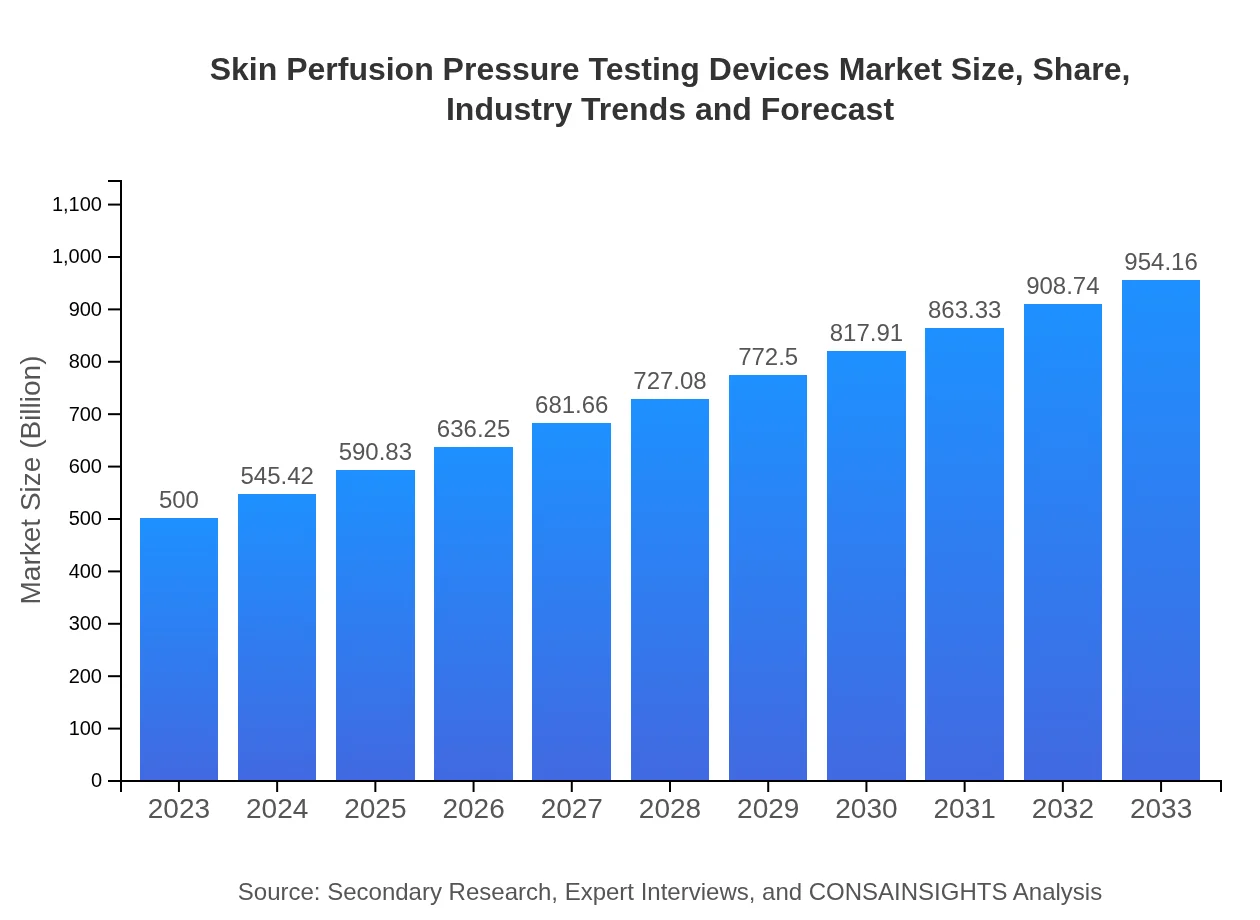
| 2023 Market Size | $500.00 Million |
| CAGR (2023-2033) | 6.5% |
| 2033 Market Size | $954.16 Million |
| Top Companies | Huntleigh Healthcare, MediGoal Group, Stryker Corporation, NITA Medical |
| Last Modified Date | 31 January 2026 |
Skin Perfusion Pressure Testing Devices Market Overview
Customize Skin Perfusion Pressure Testing Devices Market Report market research report
- ✔ Get in-depth analysis of Skin Perfusion Pressure Testing Devices market size, growth, and forecasts.
- ✔ Understand Skin Perfusion Pressure Testing Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Skin Perfusion Pressure Testing Devices
What is the Market Size & CAGR of Skin Perfusion Pressure Testing Devices market in 2023?
Skin Perfusion Pressure Testing Devices Industry Analysis
Skin Perfusion Pressure Testing Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Skin Perfusion Pressure Testing Devices Market Analysis Report by Region
Europe Skin Perfusion Pressure Testing Devices Market Report:
Europe's market is expected to increase significantly from $180.50 million in 2023 to $344.45 million by 2033 due to the emphasis on research and advanced clinical practices in the wound care sector.Asia Pacific Skin Perfusion Pressure Testing Devices Market Report:
In the Asia Pacific region, the Skin Perfusion Pressure Testing Devices market is projected to grow from $90.95 million in 2023 to $173.56 million by 2033, reflecting an increasing focus on advanced healthcare solutions and expanding healthcare infrastructure.North America Skin Perfusion Pressure Testing Devices Market Report:
North America dominates the market, growing from $168.50 million in 2023 to $321.55 million by 2033, supported by higher healthcare spending and an established healthcare infrastructure keen on adopting innovative technologies.South America Skin Perfusion Pressure Testing Devices Market Report:
The South American market, although smaller, is on the rise, with a growth from $47.95 million in 2023 to $91.50 million by 2033. This growth is mainly driven by rising diabetes incidences and associated healthcare visibility improvements.Middle East & Africa Skin Perfusion Pressure Testing Devices Market Report:
In the Middle East and Africa, the market for Skin Perfusion Pressure Testing Devices is anticipated to rise from $12.10 million to $23.09 million between 2023 and 2033, driven by improving healthcare standards and a burgeoning awareness of chronic illness management.Tell us your focus area and get a customized research report.
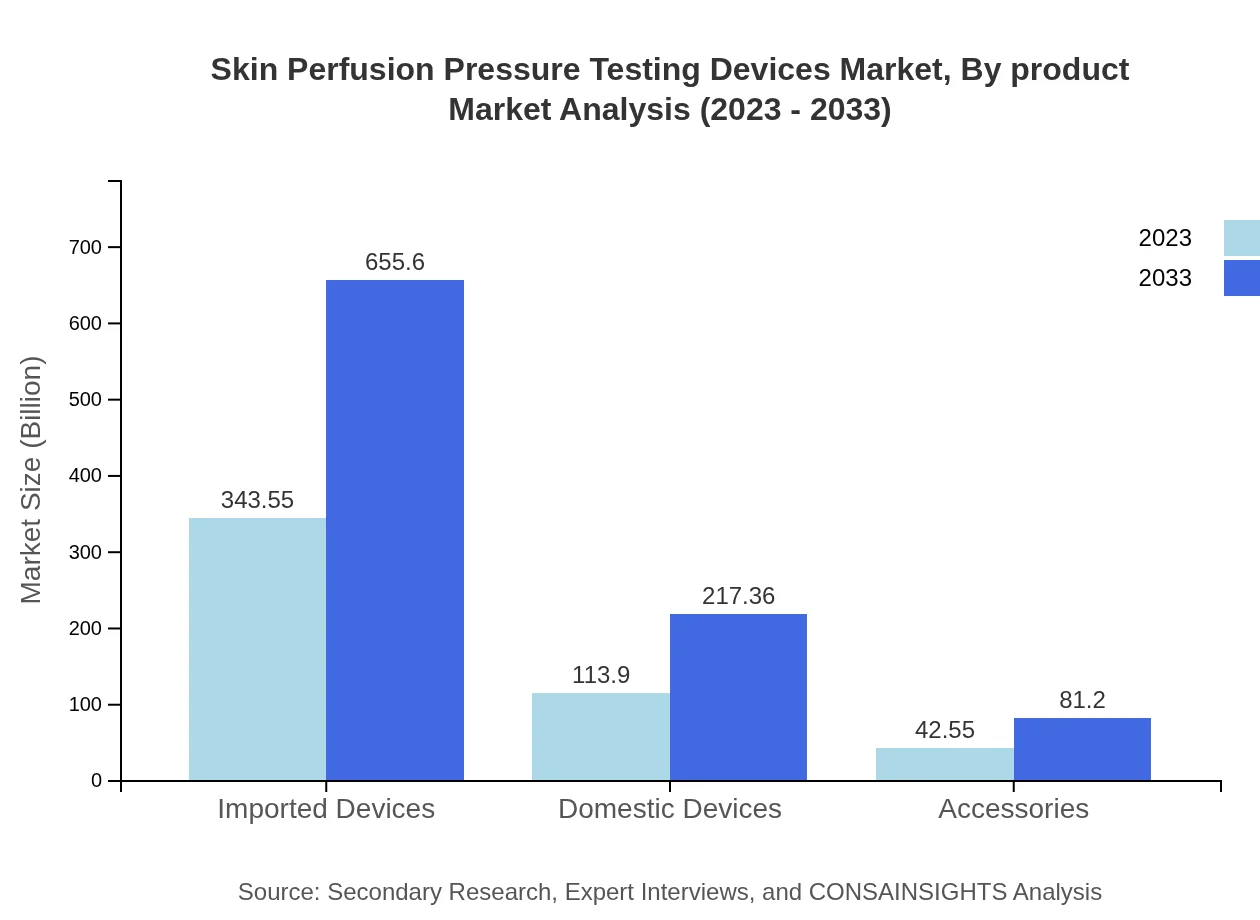
Skin Perfusion Pressure Testing Devices Market Analysis By Product
The product segment of Skin Perfusion Pressure Testing Devices is divided into imported and domestic devices, with imported devices dominating the market at approximately 68.71% market share in 2023, worth roughly $343.55 million, projected to increase to $655.60 million by 2033. Domestic devices are also growing, with a market size of $113.90 million in 2023, expected to reach $217.36 million by 2033.
Skin Perfusion Pressure Testing Devices Market Analysis By Application
The applications of Skin Perfusion Pressure Testing Devices are crucial in various sectors, predominantly hospitals, which account for a significant share of the market. Hospitals valued at $343.55 million in 2023 are predicted to see revenues skyrocket to $655.60 million by 2033. Clinics and research institutes also contribute meaningfully, with shares of 22.78% and 8.51% respectively.
Skin Perfusion Pressure Testing Devices Market Analysis By End User
End-users of Skin Perfusion Pressure Testing Devices are primarily healthcare institutes, including hospitals and clinics. These sectors demonstrated a robust demand, driven by the necessity for precision in wound care management, supporting an overall market growth resulting in significant adoption rates.
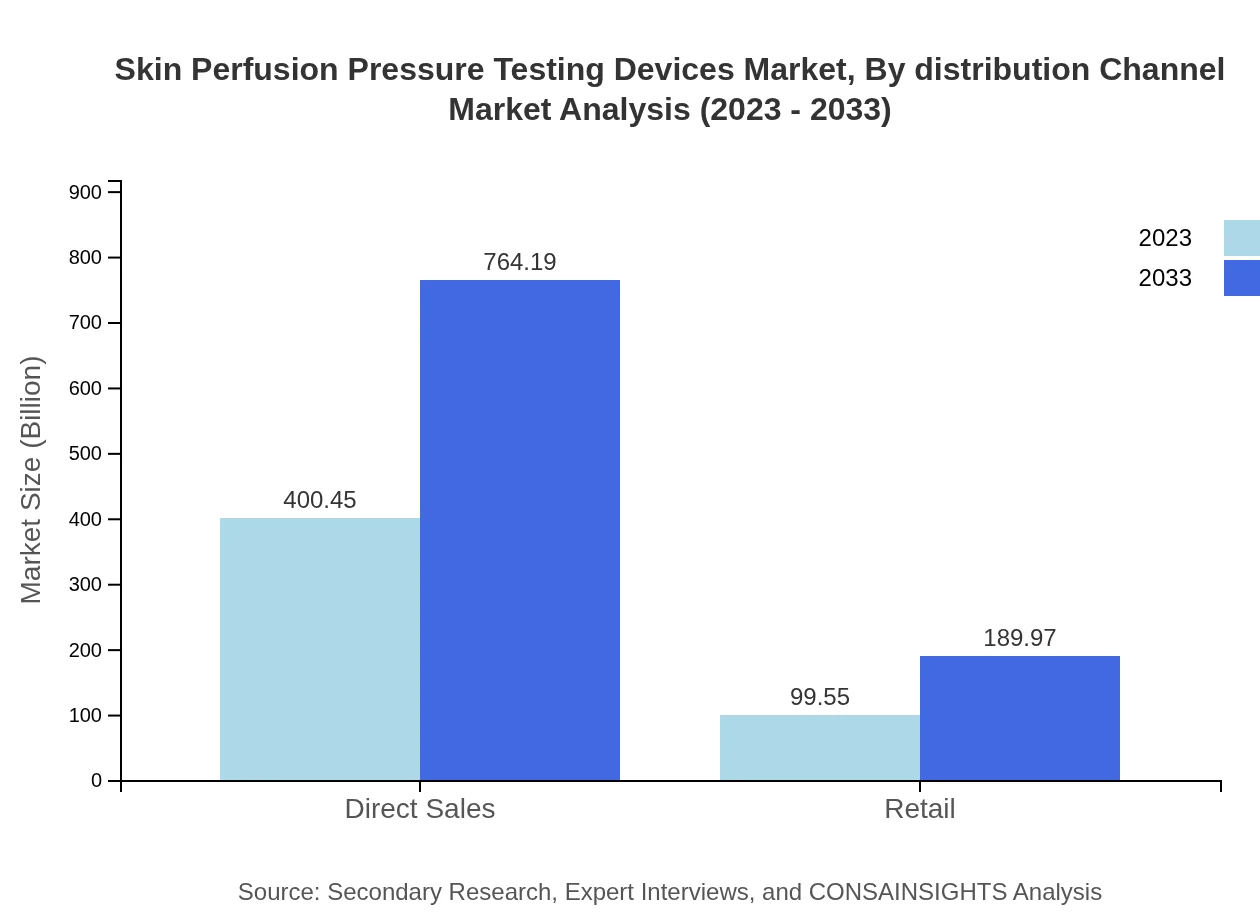
Skin Perfusion Pressure Testing Devices Market Analysis By Distribution Channel
Skin Perfusion Pressure Testing Devices are distributed primarily through direct sales and retail channels. Direct sales, holding 80.09% of the market share in 2023, are poised to grow from $400.45 million to $764.19 million by 2033. Retail also plays a significant role with around 19.91% market share.
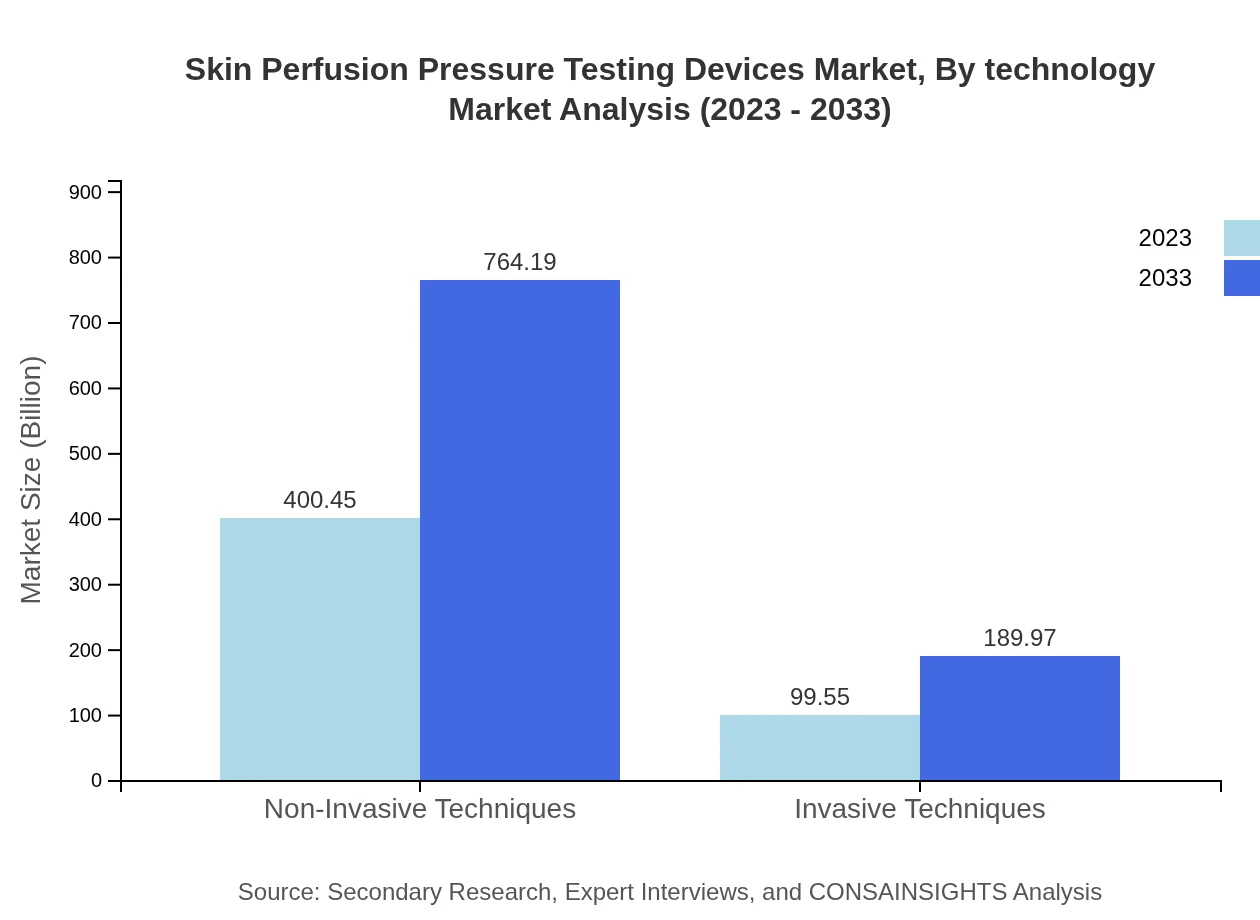
Skin Perfusion Pressure Testing Devices Market Analysis By Technology
In terms of technology, non-invasive devices hold a substantial stake in the market, comprising over 80.09% of the total market volume in 2023. This segment is expected to grow from $400.45 million to $764.19 million by 2033, indicating a significant demand driven by the shift towards safer monitoring solutions.
Skin Perfusion Pressure Testing Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Skin Perfusion Pressure Testing Devices Industry
Huntleigh Healthcare:
A leading manufacturer of medical devices focusing on non-invasive diagnostic tools for vascular assessments and wound care management.MediGoal Group:
Specializes in advanced solutions for pressure ulcer management, offering a comprehensive range of perfusion pressure testing devices and protocols.Stryker Corporation:
Globally recognized for its extensive investment in medical technologies and products, Stryker provides state-of-the-art solutions for patient care, including perfusion pressure devices.NITA Medical:
Innovative player in the medical device industry, focusing on the development of advanced measuring devices for skin perfusion and related applications.We're grateful to work with incredible clients.









FAQs
What is the market size of skin perfusion pressure testing devices?
The global skin perfusion pressure testing devices market is valued at approximately $500 million in 2023 and is projected to grow at a CAGR of 6.5%, signaling a robust increase in demand through 2033.
What are the key market players or companies in this skin perfusion pressure testing devices industry?
Key players in the skin perfusion pressure testing devices market include major medical device manufacturers and healthcare companies specializing in non-invasive monitoring, which contribute to advancements in product quality and innovation.
What are the primary factors driving the growth in the skin perfusion pressure testing devices industry?
Growth drivers include an increase in diabetic foot ulcers, a rise in outpatient procedures, and technological advancements in device accuracy and portability, enhancing the demand for effective monitoring solutions.
Which region is the fastest Growing in the skin perfusion pressure testing devices?
The Asia-Pacific region is anticipated to be the fastest-growing market, expanding from $90.95 million in 2023 to an estimated $173.56 million by 2033, driven by increased healthcare access and investment.
Does ConsaInsights provide customized market report data for the skin perfusion pressure testing devices industry?
Yes, ConsaInsights offers tailored market report data to meet specific client needs, ensuring comprehensive insights into niche segments and localized demand trends within the skin perfusion pressure testing devices market.
What deliverables can I expect from this skin perfusion pressure testing devices market research project?
Deliverables include detailed market analysis reports, forecasts, competitive landscape assessments, regional insights, and segment data, providing a holistic view of the current market and future opportunities.
What are the market trends of skin perfusion pressure testing devices?
Current trends include the increasing adoption of non-invasive techniques, advancements in device technology, a shift towards home healthcare, and a growing emphasis on early detection of vascular insufficiencies among patients.