Specimen Validity Testing Market Report
Published Date: 31 January 2026 | Report Code: specimen-validity-testing
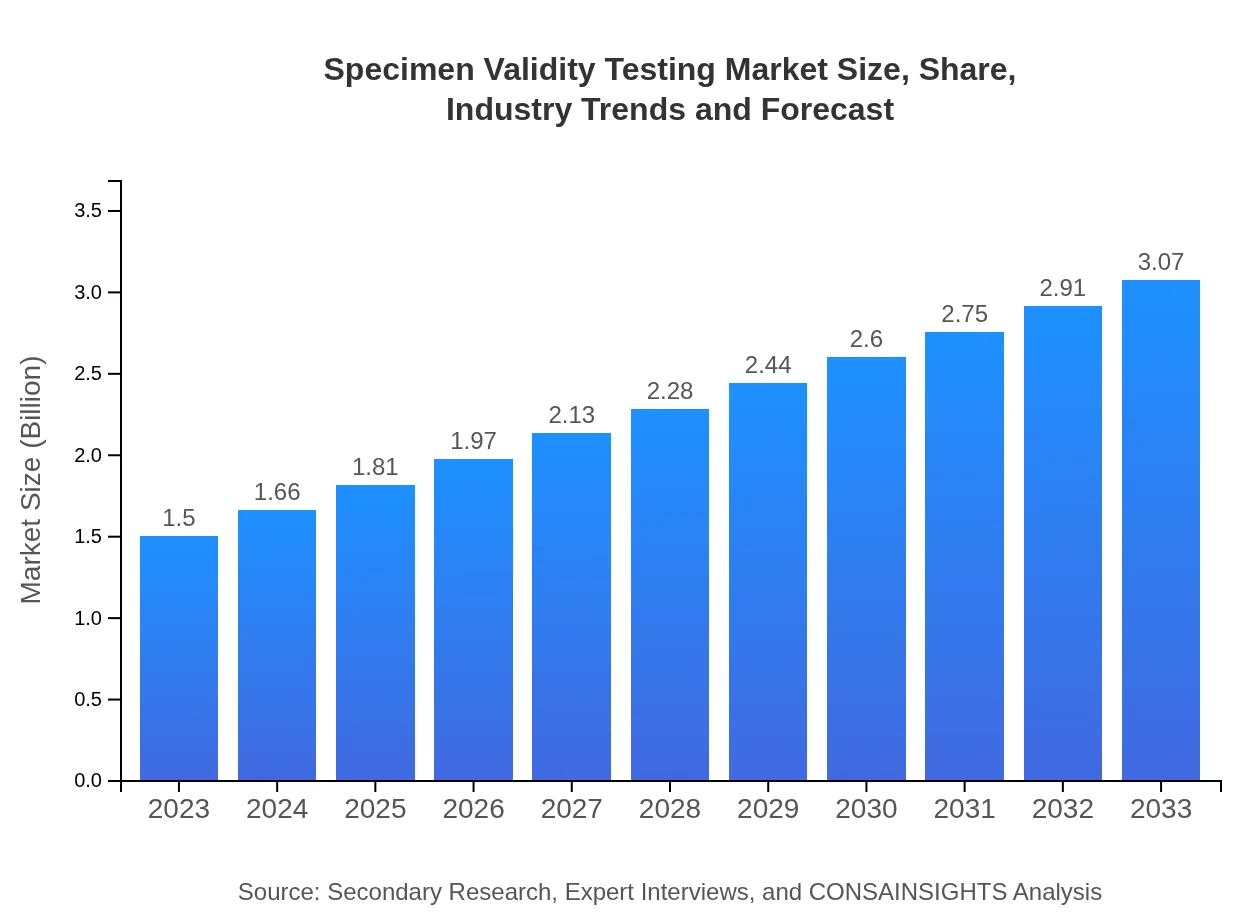
Specimen Validity Testing Market Size, Share, Industry Trends and Forecast to 2033
This report delves into the Specimen Validity Testing market, detailing insights into current market conditions, trends, and forecasts from 2023 to 2033, while providing comprehensive analysis across various segments and regions.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $1.50 Billion |
| CAGR (2023-2033) | 7.2% |
| 2033 Market Size | $3.07 Billion |
| Top Companies | Quest Diagnostics, LabCorp, Innovative Bioresearch, Roche Diagnostics |
| Last Modified Date | 31 January 2026 |
Specimen Validity Testing Market Overview
Customize Specimen Validity Testing Market Report market research report
- ✔ Get in-depth analysis of Specimen Validity Testing market size, growth, and forecasts.
- ✔ Understand Specimen Validity Testing's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Specimen Validity Testing
What is the Market Size & CAGR of Specimen Validity Testing market in 2023?
Specimen Validity Testing Industry Analysis
Specimen Validity Testing Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Specimen Validity Testing Market Analysis Report by Region
Europe Specimen Validity Testing Market Report:
In Europe, the market is expected to grow from $0.42 billion in 2023 to $0.87 billion by 2033. The regulatory focus on safety and compliance, combined with an increasing number of drug testing facilities, bolsters this segment's expansion.Asia Pacific Specimen Validity Testing Market Report:
In the Asia Pacific region, the Specimen Validity Testing market is projected to grow from $0.29 billion in 2023 to $0.58 billion by 2033. The growth is attributed to increasing drug testing initiatives and improving healthcare infrastructure supporting testing advancements.North America Specimen Validity Testing Market Report:
North America is anticipated to witness substantial growth, with the market size expected to rise from $0.56 billion in 2023 to $1.14 billion by 2033. The region's stringent regulations regarding workplace drug testing and growing prevalence of substance abuse contribute significantly to this demand.South America Specimen Validity Testing Market Report:
The South American market for Specimen Validity Testing is forecasted to expand from $0.15 billion in 2023 to $0.31 billion by 2033. Notably, the rising awareness of drug abuse and regulatory mandates are driving demand for reliable testing solutions in this region.Middle East & Africa Specimen Validity Testing Market Report:
The Middle East and Africa market is set to increase from $0.08 billion in 2023 to $0.17 billion by 2033, driven by growing healthcare investments and the rising need for drug testing in various sectors such as workplace and clinical applications.Tell us your focus area and get a customized research report.
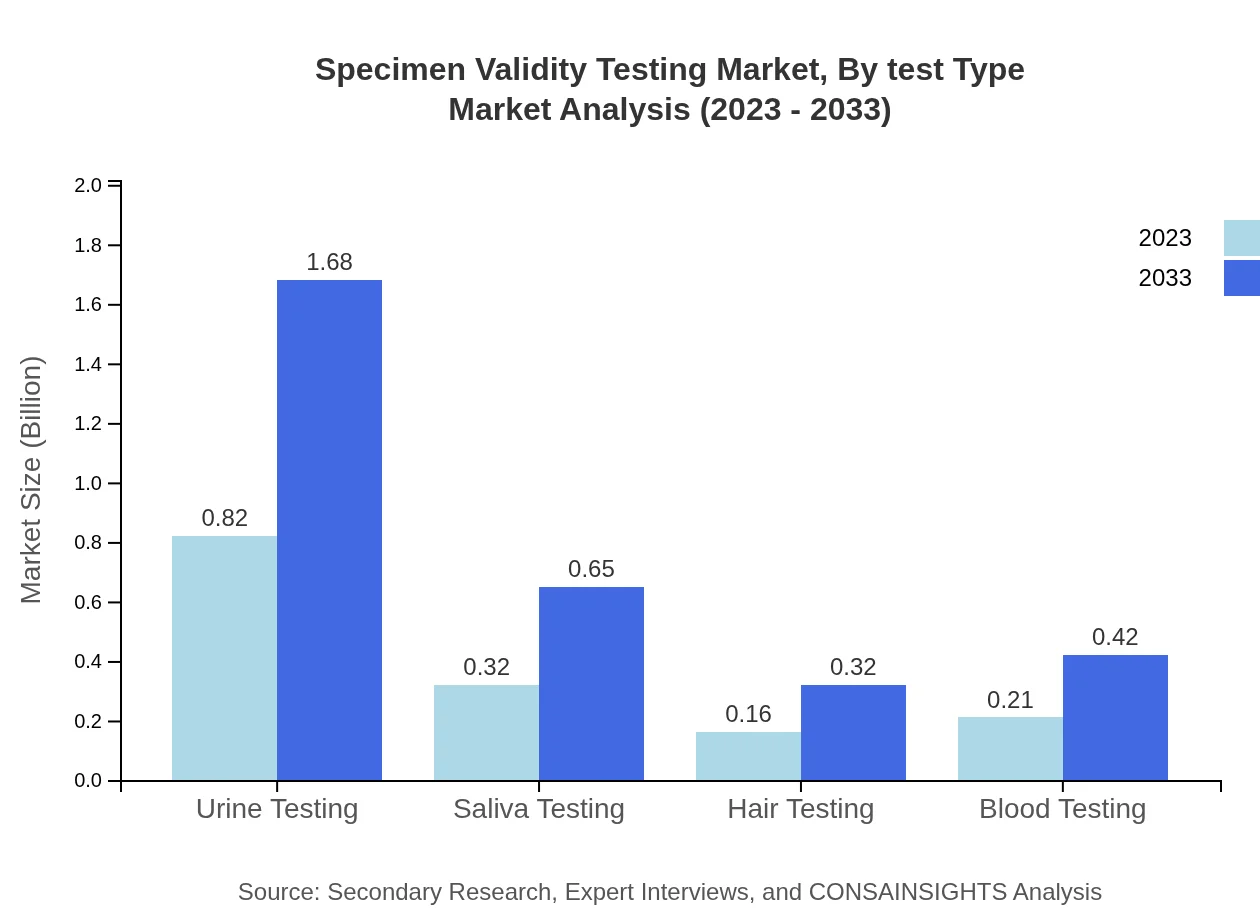
Specimen Validity Testing Market Analysis By Test Type
Currently, urine testing dominates the Specimen Validity Testing market, representing 54.77% market share in 2023 and expected to remain consistent through 2033. Saliva testing follows at 21.04% market share, with significant growth anticipated. Hair and blood testing constitute crucial segments, reflecting increasing diversification in testing methods.
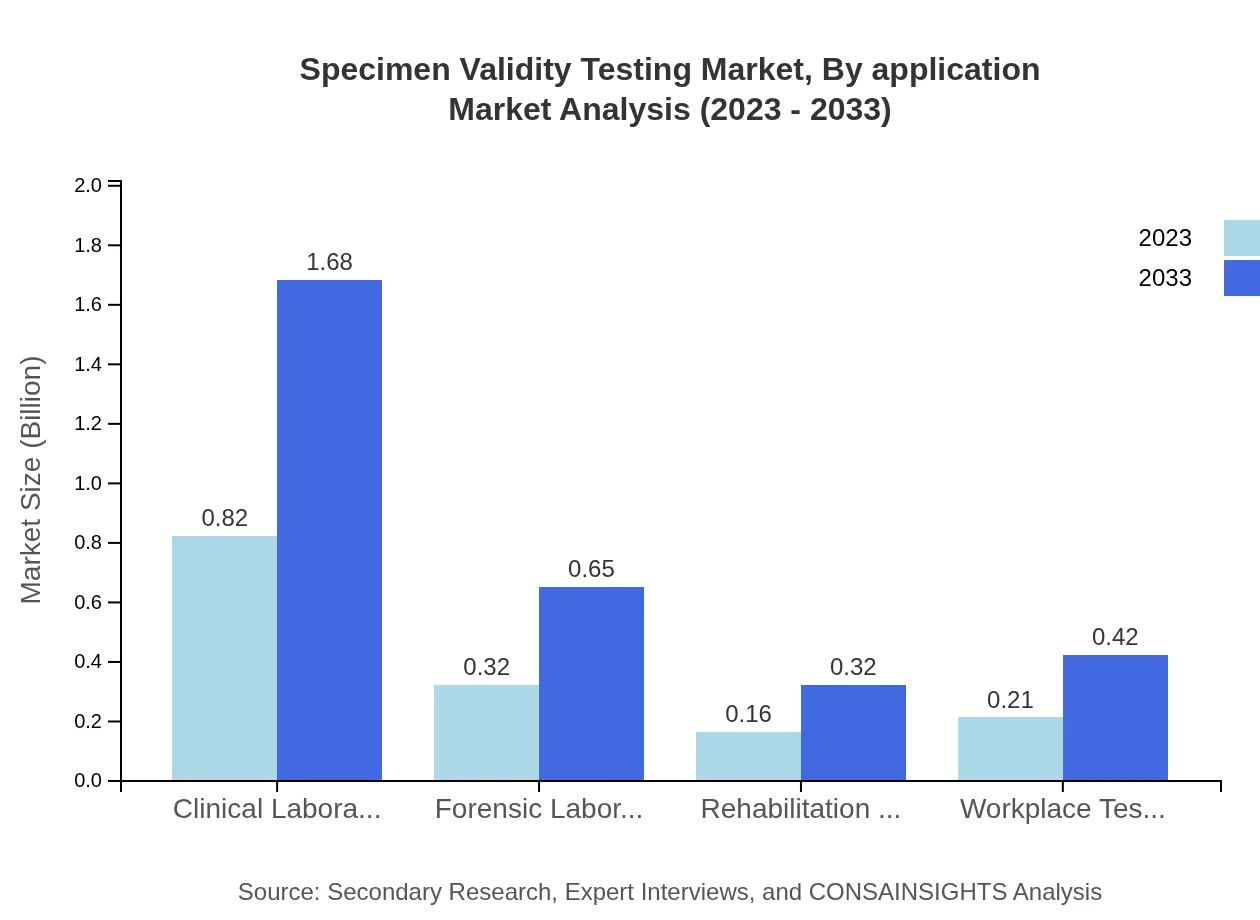
Specimen Validity Testing Market Analysis By Application
Hospitals represent the largest application segment, accounting for a 54.77% market share in 2023, with a projected growth to 54.77% by 2033. Diagnostic centers and clinical laboratories also contribute significantly, serving both routine and critical testing applications.
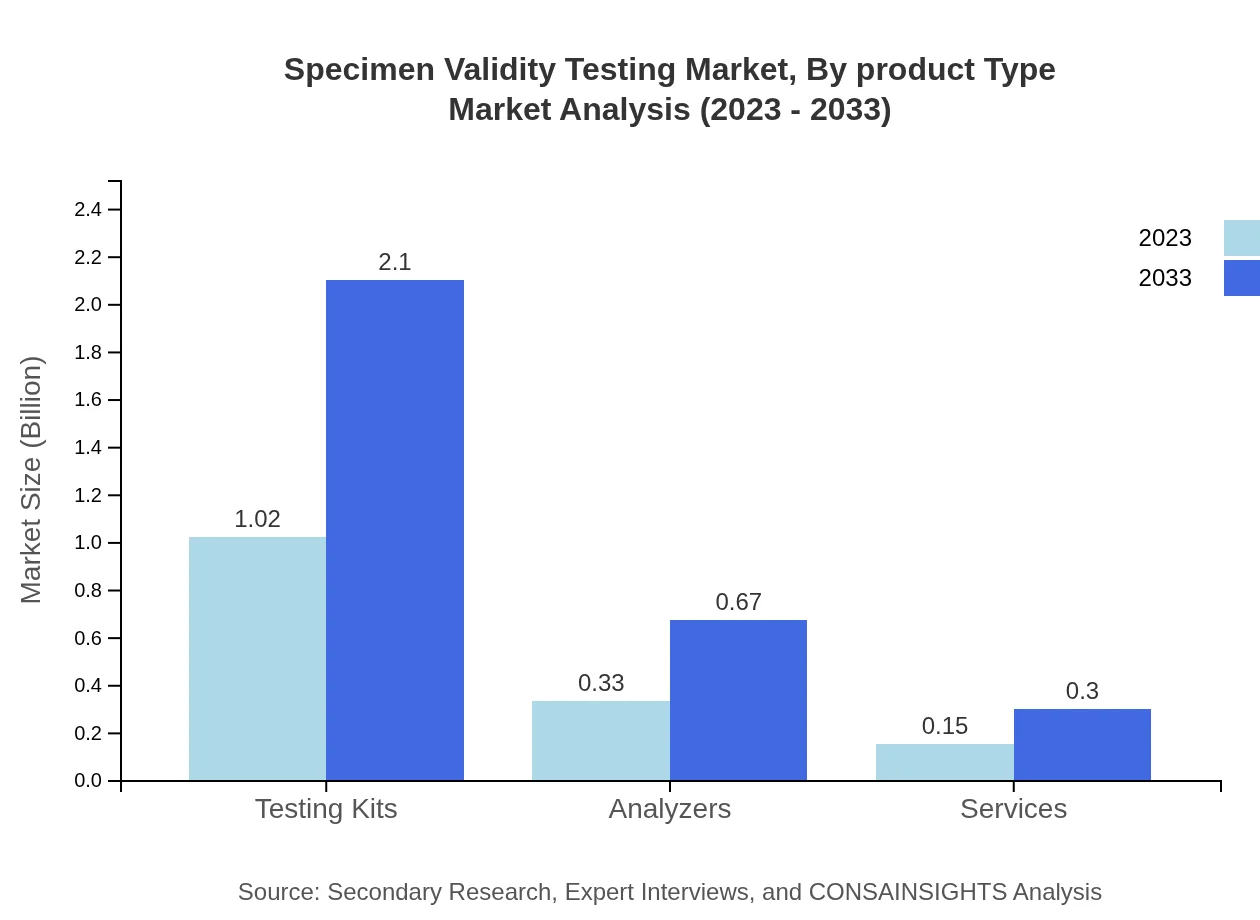
Specimen Validity Testing Market Analysis By Product Type
Testing kits hold the largest share of the Specimen Validity Testing market at 68.33% in 2023, with expected growth due to continuous innovation. Analyzers and services are also crucial as healthcare facilities adopt comprehensive testing solutions to meet regulatory standards.
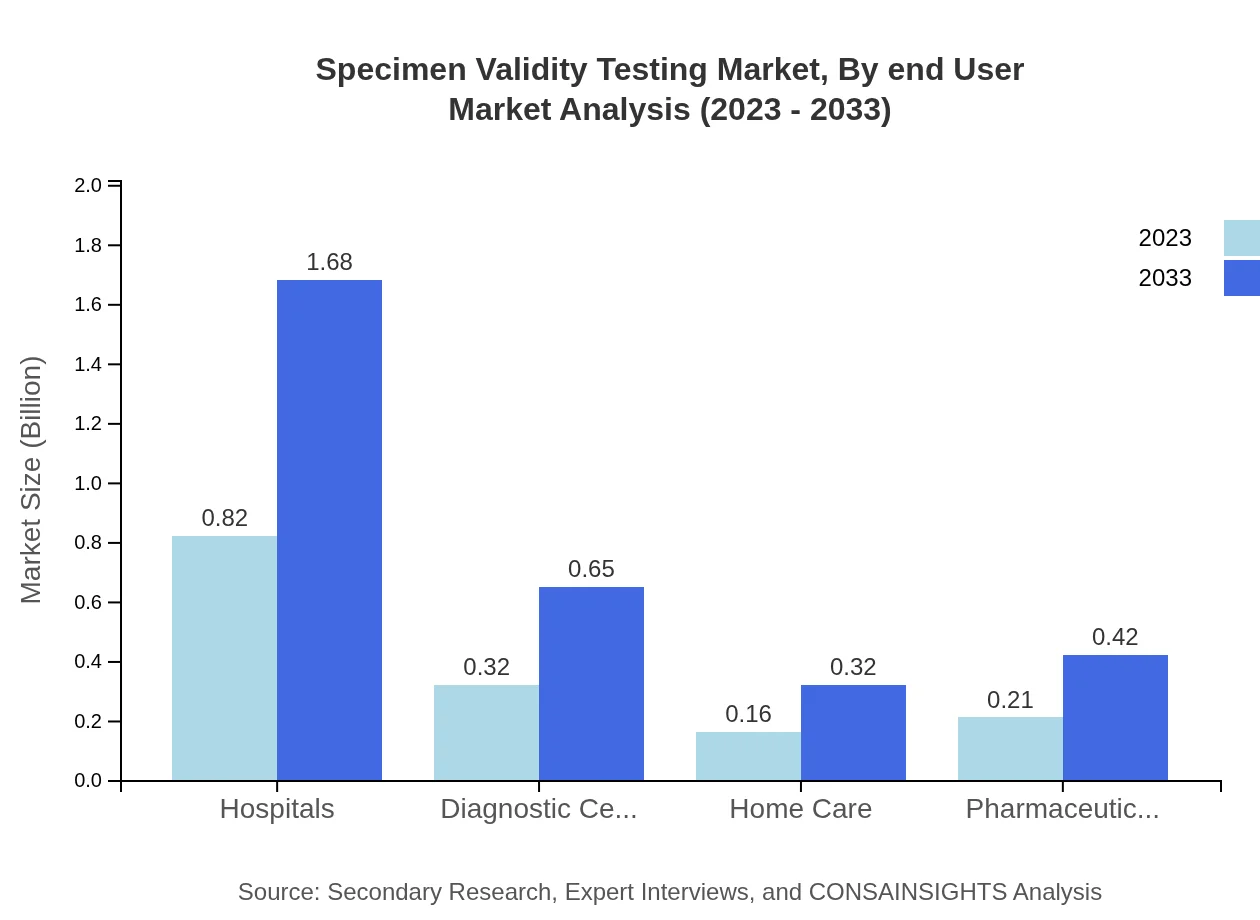
Specimen Validity Testing Market Analysis By End User
End-user segments include hospitals, diagnostic centers, and forensic laboratories, among others. Hospitals accounted for a significant market share in 2023. Workplace testing is also growing as organizations aim to enforce substance abuse policies.
Specimen Validity Testing Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Specimen Validity Testing Industry
Quest Diagnostics:
Quest Diagnostics is a leading provider of diagnostic information services. They offer a range of testing solutions in the specimen validity testing space, emphasizing innovations in drug testing methodologies.LabCorp:
Laboratory Corporation of America Holdings (LabCorp) provides comprehensive laboratory services, including specimen validity testing. Their commitment to advanced technologies and customer-focused solutions positions them as a key player in the market.Innovative Bioresearch:
Innovative Bioresearch is renowned for its advancements in testing kits and solutions specifically designed for specimen validity testing, contributing to increasing accuracy and reliability.Roche Diagnostics:
Roche Diagnostics delivers a wide range of diagnostic tests and services, focusing on innovation and quality that enhances the specimen validity testing market through advanced analyzers and diagnostic kits.We're grateful to work with incredible clients.









FAQs
What is the market size of specimen Validity Testing?
The specimen validity testing market is currently valued at $1.5 billion, with an expected CAGR of 7.2% over the next decade, projecting robust growth in demand for reliability in diagnostic processes.
What are the key market players or companies in this specimen Validity Testing industry?
Key players in the specimen validity testing market include Abbott Laboratories, Quest Diagnostics, LabCorp, and Thermo Fisher Scientific, all competing to enhance product quality and innovation to meet growing market needs.
What are the primary factors driving the growth in the specimen Validity Testing industry?
Growth in the specimen validity testing sector is driven by rising drug testing regulations, an increase in workplace safety concerns, and advancements in testing technologies enhancing test accuracy and reliability.
Which region is the fastest Growing in the specimen Validity Testing?
Europe is currently the fastest-growing region in the specimen validity testing market, expected to grow from $0.42 billion in 2023 to $0.87 billion by 2033, reflecting driven demand for enhanced healthcare regulations.
Does ConsaInsights provide customized market report data for the specimen Validity Testing industry?
Yes, ConsaInsights offers customized market report data specifically tailored for the specimen validity testing industry, providing clients with insights that meet their strategic planning needs.
What deliverables can I expect from this specimen Validity Testing market research project?
Deliverables from this project include detailed market analysis, segmentation data, regional insights, and recommendations for strategic initiatives, ensuring informed decision-making for stakeholders.
What are the market trends of specimen Validity Testing?
Current trends in specimen validity testing highlight increasing adoption of rapid testing technologies, enhancements in testing kits, and a shift towards more comprehensive drug and substance testing protocols across various sectors.