Spinal Muscular Atrophy Treatment Market Report
Published Date: 31 January 2026 | Report Code: spinal-muscular-atrophy-treatment
Spinal Muscular Atrophy Treatment Market Size, Share, Industry Trends and Forecast to 2033
This report provides comprehensive insights into the Spinal Muscular Atrophy Treatment market, focusing on market trends, growth forecasts, and segmentation strategies from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
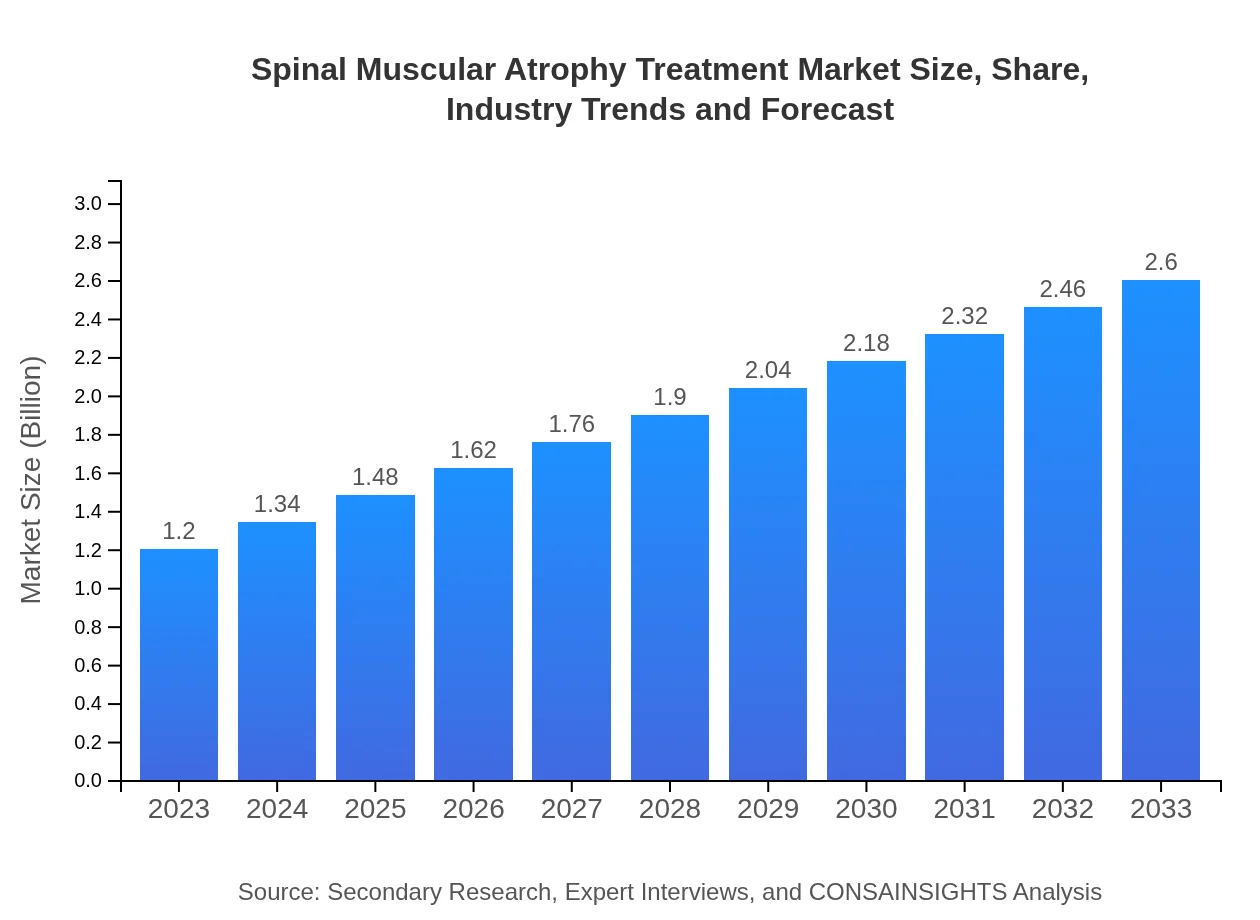
| 2023 Market Size | $1.20 Billion |
| CAGR (2023-2033) | 7.8% |
| 2033 Market Size | $2.60 Billion |
| Top Companies | Novartis, Biogen, Roche, Bayer |
| Last Modified Date | 31 January 2026 |
Spinal Muscular Atrophy Treatment Market Overview
Customize Spinal Muscular Atrophy Treatment Market Report market research report
- ✔ Get in-depth analysis of Spinal Muscular Atrophy Treatment market size, growth, and forecasts.
- ✔ Understand Spinal Muscular Atrophy Treatment's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Spinal Muscular Atrophy Treatment
What is the Market Size & CAGR of Spinal Muscular Atrophy Treatment market in 2023?
Spinal Muscular Atrophy Treatment Industry Analysis
Spinal Muscular Atrophy Treatment Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Spinal Muscular Atrophy Treatment Market Analysis Report by Region
Europe Spinal Muscular Atrophy Treatment Market Report:
The European market for SMA treatment is projected to grow from $0.32 billion in 2023 to $0.70 billion by 2033. The growing demand for innovative therapies and the supportive regulatory environment contribute to this growth.Asia Pacific Spinal Muscular Atrophy Treatment Market Report:
The Asia Pacific region is witnessing a significant rise in the SMA treatment market, expected to grow from $0.26 billion in 2023 to $0.57 billion by 2033. This growth is fueled by increasing awareness of SMA, improved access to healthcare facilities, and ongoing research activities aimed at developing effective treatments.North America Spinal Muscular Atrophy Treatment Market Report:
North America holds the largest market share in the Spinal Muscular Atrophy Treatment segment, with the market size expected to grow from $0.39 billion in 2023 to $0.84 billion by 2033. This is primarily due to the presence of key market players and advanced research facilities catering to SMA.South America Spinal Muscular Atrophy Treatment Market Report:
In South America, the SMA treatment market is poised for growth, projected to rise from $0.11 billion in 2023 to $0.23 billion by 2033. The enhancements in healthcare infrastructure and rising public and private sector investments are anticipated to drive market expansion in this region.Middle East & Africa Spinal Muscular Atrophy Treatment Market Report:
The SMA treatment market in the Middle East and Africa is anticipated to increase from $0.12 billion in 2023 to $0.26 billion by 2033, largely driven by improvements in healthcare access and increasing awareness of genetic disorders.Tell us your focus area and get a customized research report.
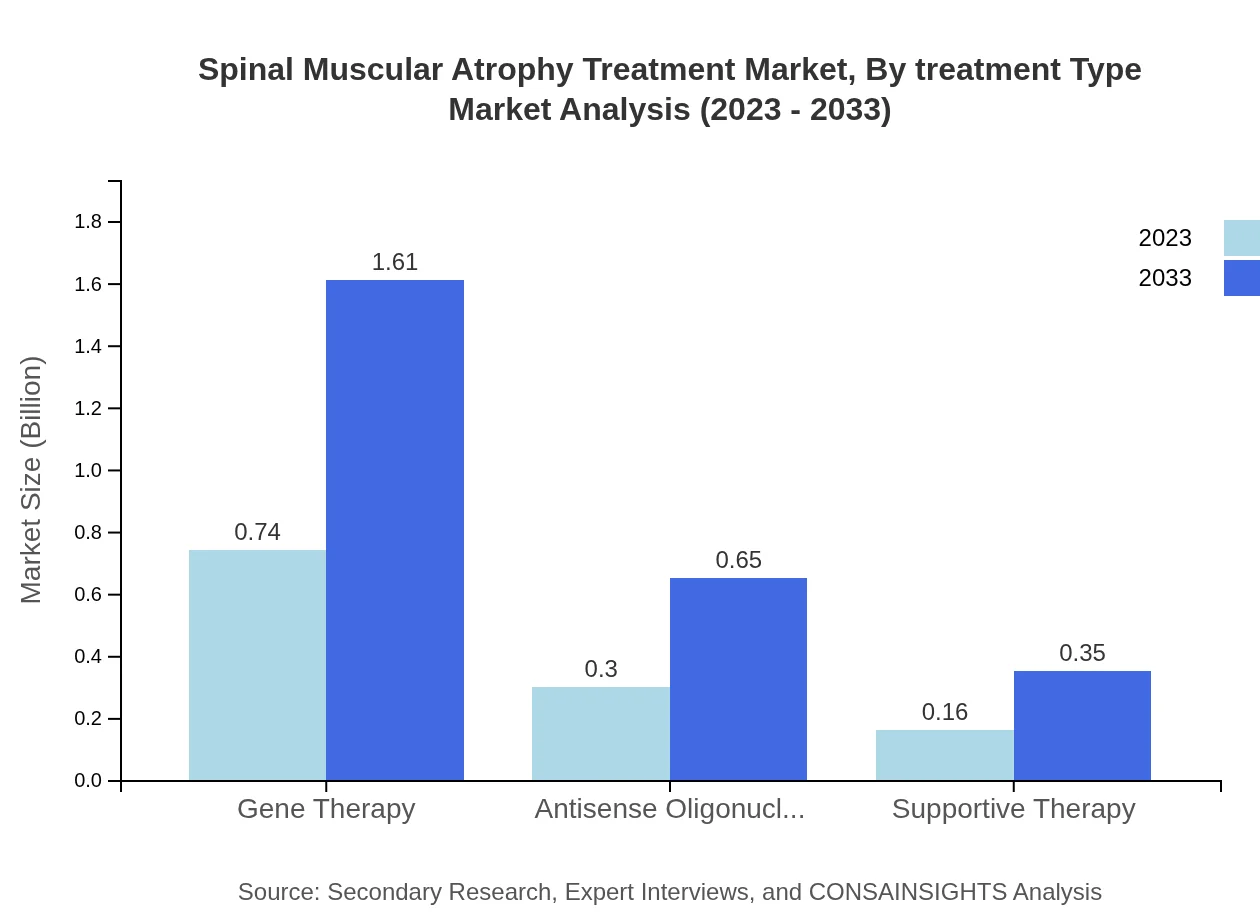
Spinal Muscular Atrophy Treatment Market Analysis By Treatment Type
The Spinal Muscular Atrophy Treatment market is significantly dominated by gene therapy, projected to grow from $0.74 billion in 2023 to $1.61 billion by 2033. Antisense oligonucleotides also exhibit substantial growth, moving from $0.30 billion to $0.65 billion in the same timeframe. Supportive therapies remain relevant, contributing from $0.16 billion to $0.35 billion in market size. Monotherapy and combination therapy strategies are essential components of treatment regimens, catering to patient-specific needs.
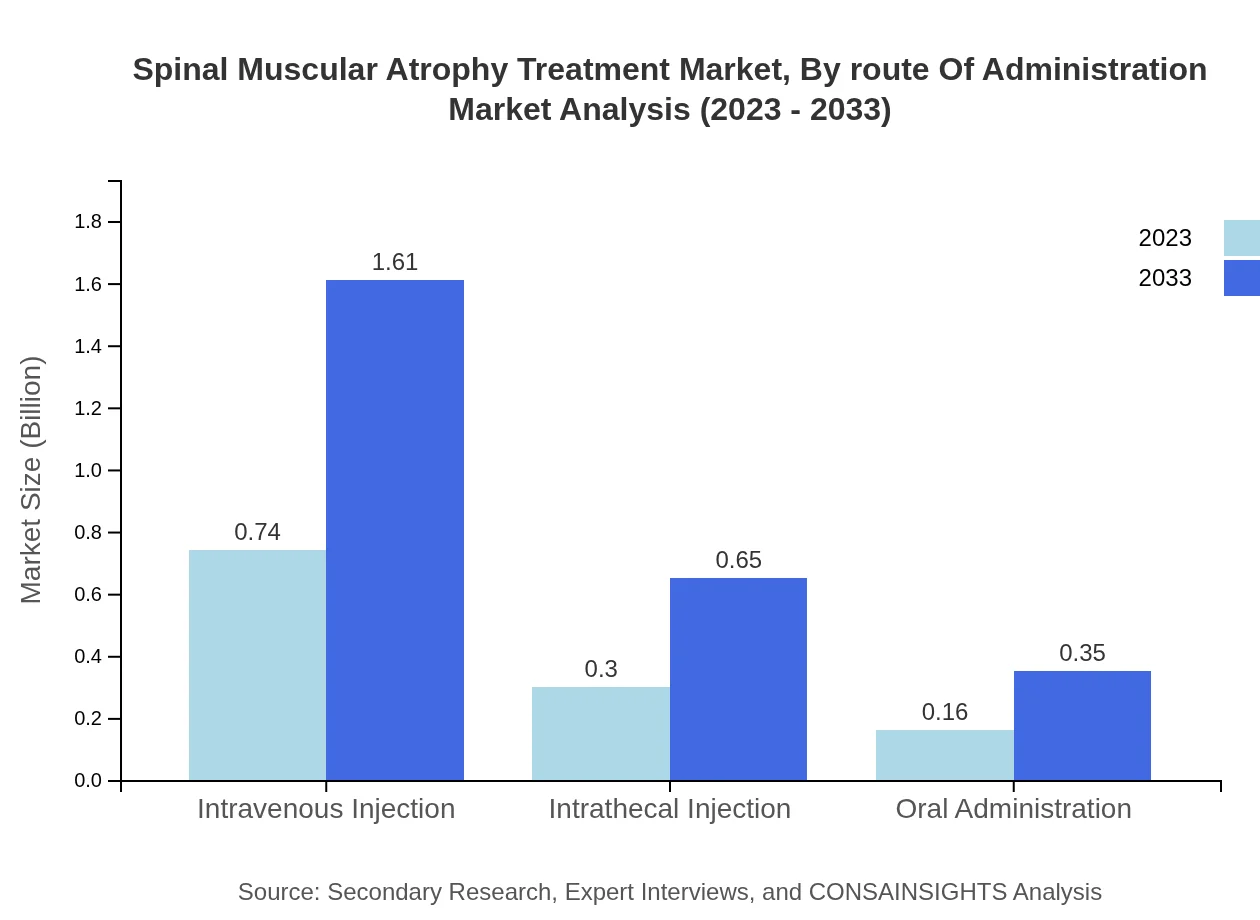
Spinal Muscular Atrophy Treatment Market Analysis By Route Of Administration
Administration routes for SMA treatments include intravenous injections, intrathecal injections, and oral administration. Intravenous injections dominate the market, projected to rise from $0.74 billion in 2023 to $1.61 billion by 2033, maintaining a strong share in effective delivery methods. Intrathecal injections follow, expected to grow from $0.30 billion to $0.65 billion, whereas oral administration will expand from $0.16 billion to $0.35 billion, providing options for patient convenience.
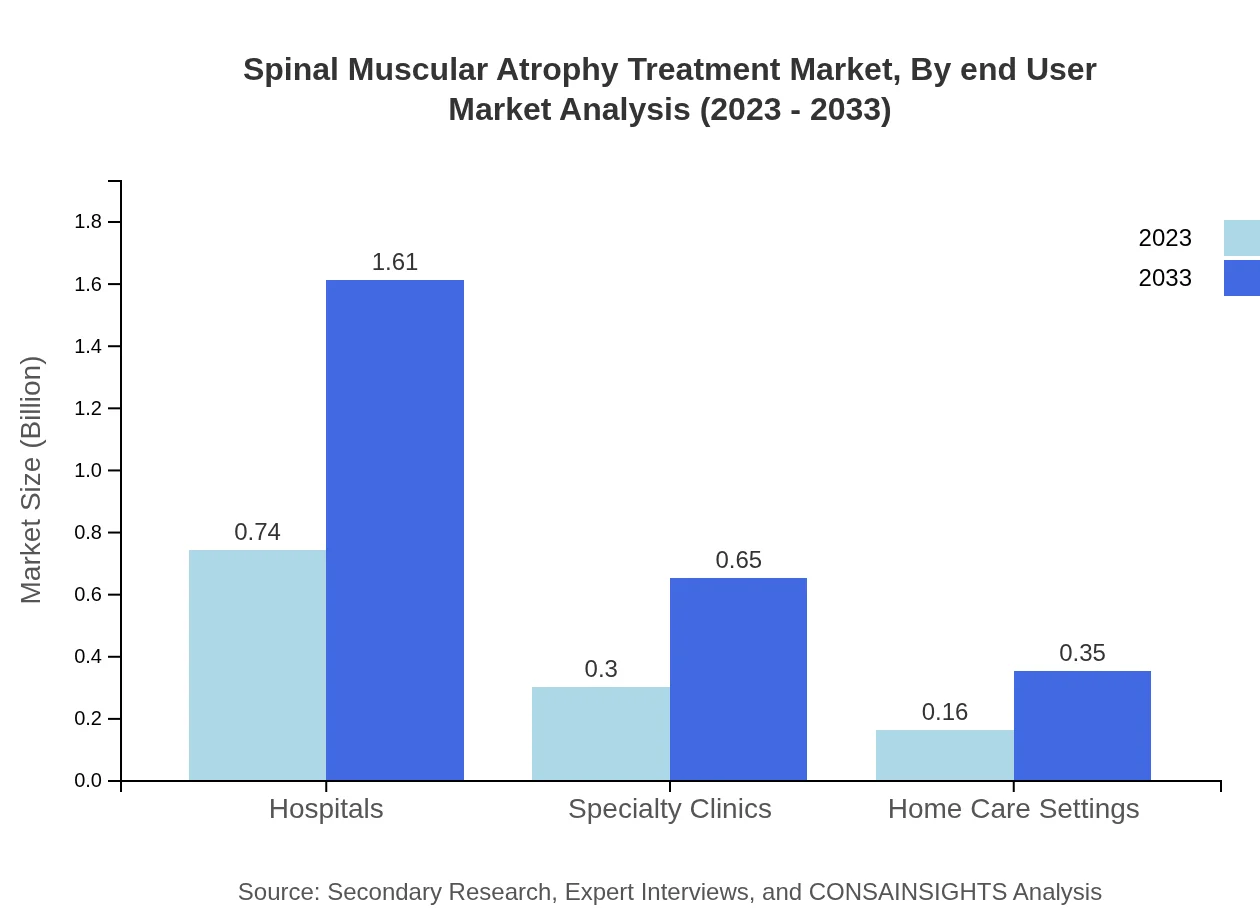
Spinal Muscular Atrophy Treatment Market Analysis By End User
Hospitals are the leading end-user segment in the SMA treatment market, accounting for a significant portion with a market size estimated at $0.74 billion in 2023, expected to reach $1.61 billion by 2033. Specialty clinics are important with an upward trend from $0.30 billion to $0.65 billion. Home care settings provide an equally important role, increasing from $0.16 billion to $0.35 billion, indicating a shift towards more personalized care.
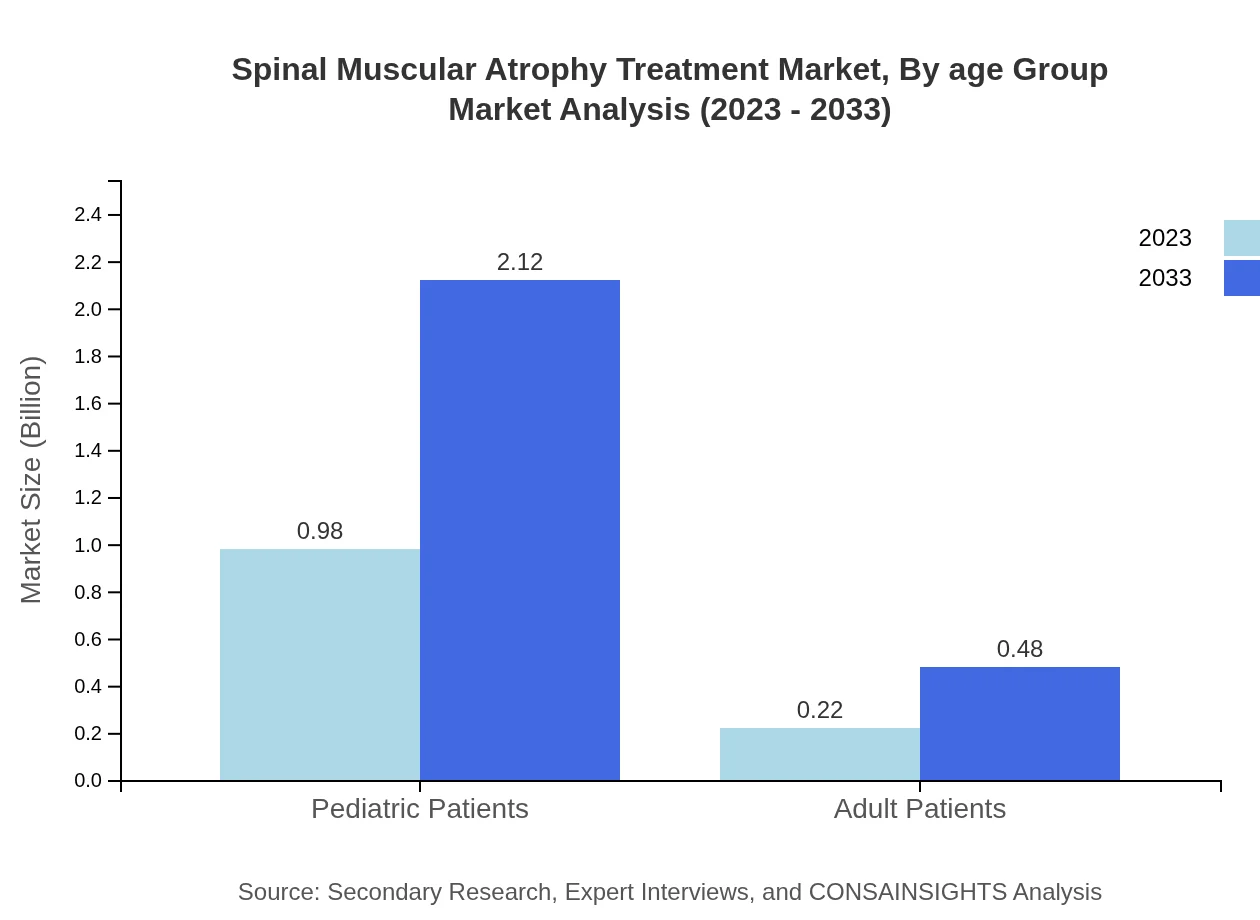
Spinal Muscular Atrophy Treatment Market Analysis By Age Group
The pediatric population dominates the SMA treatment market, forecasted to grow from $0.98 billion in 2023 to $2.12 billion by 2033. In contrast, adults represent a smaller segment, projected to increase from $0.22 billion to $0.48 billion. Tailored treatment strategies and continuity of care are crucial for both demographics.
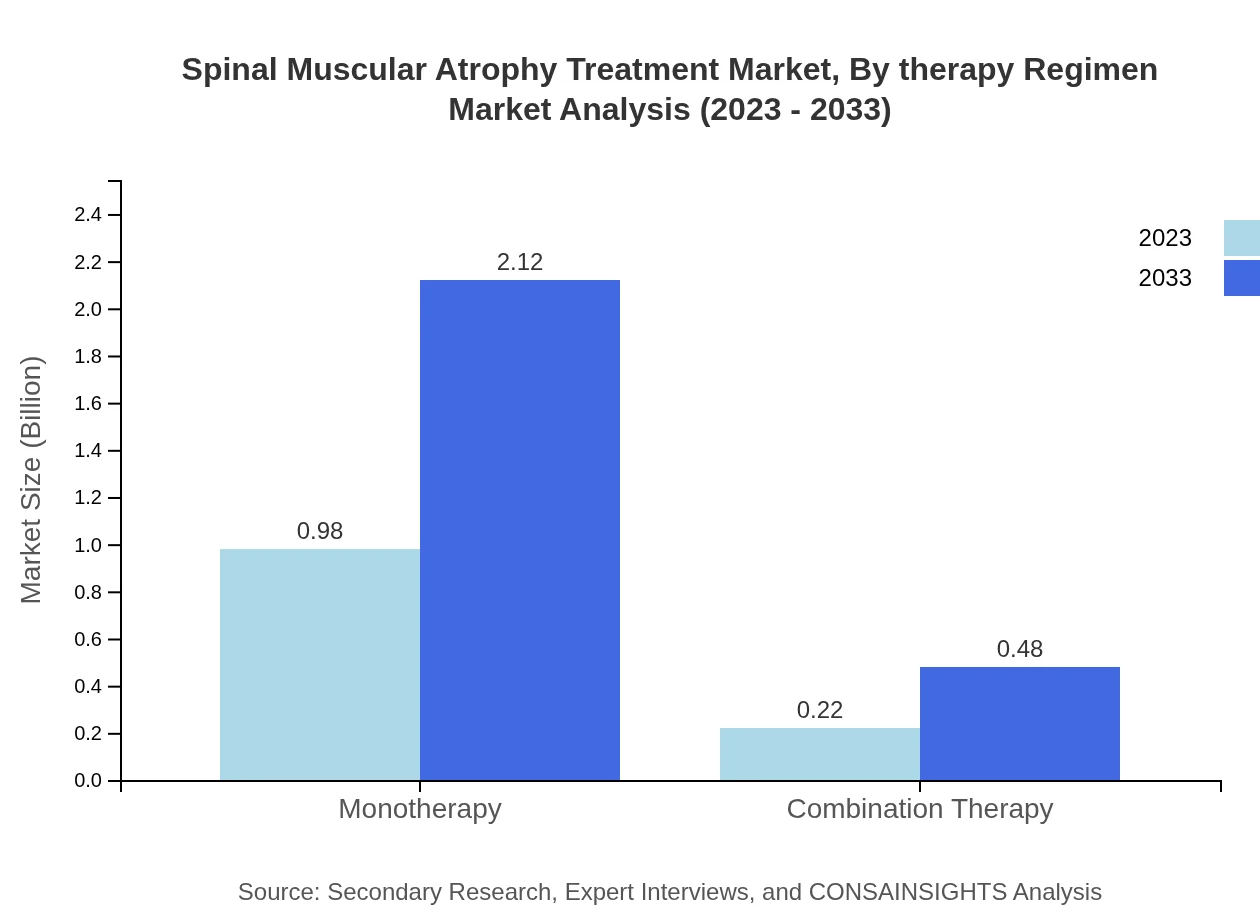
Spinal Muscular Atrophy Treatment Market Analysis By Therapy Regimen
Monotherapy remains the preferred treatment regimen, holding significant market share and expected growth from $0.98 billion to $2.12 billion. Combination therapies cater to specific patient needs, poised to grow from $0.22 billion to $0.48 billion. The focus on personalized therapies enhances the patient outcome prospects.
Spinal Muscular Atrophy Treatment Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Spinal Muscular Atrophy Treatment Industry
Novartis:
A leading player in the SMA treatment market known for its innovative gene therapy product, Zolgensma, which is a revolutionary treatment for children with SMA.Biogen:
Recognized for its treatment Spinraza, Biogen plays a pivotal role in addressing SMA, focusing on therapies that improve the quality of life of patients.Roche:
Roche is committed to rare disease treatments, investing in research for SMA solutions and developing novel drugs to further aid patients' health.Bayer:
Bayer is involved in innovative approaches for SMA treatment focusing on providing supportive therapies and improving methodologies for patient care.We're grateful to work with incredible clients.









FAQs
What is the market size of spinal Muscular Atrophy Treatment?
The spinal muscular atrophy treatment market is projected to reach a size of approximately $1.2 billion by 2033, with a CAGR of 7.8% from its current value. This growth indicates increasing demand for effective therapies and innovations in treatment options.
What are the key market players or companies in this spinal Muscular Atrophy Treatment industry?
The key market players in the spinal muscular atrophy treatment industry include leading biotechnology and pharmaceutical companies specializing in genetic therapies and neuromuscular disorders. Companies focusing on gene therapy and antisense oligonucleotides are particularly pivotal.
What are the primary factors driving the growth in the spinal Muscular Atrophy Treatment industry?
Growth in the spinal muscular atrophy treatment market is driven by factors like advancements in gene therapy, increasing awareness of SMA, rise in pediatric diagnoses, and the successful market introduction of novel therapies addressing the root cause of the disease.
Which region is the fastest Growing in the spinal Muscular Atrophy Treatment?
North America is the fastest-growing region in the spinal muscular atrophy treatment market, with a projected size increase from $0.39 billion in 2023 to $0.84 billion by 2033, reflecting strong healthcare infrastructure and investment in innovative therapies.
Does ConsaInsights provide customized market report data for the spinal Muscular Atrophy Treatment industry?
Yes, ConsaInsights offers customized market report data tailored to the specific needs of clients in the spinal muscular atrophy treatment industry. This enables stakeholders to gain insights relevant to their unique business or research interests.
What deliverables can I expect from this spinal Muscular Atrophy Treatment market research project?
Expect comprehensive deliverables from this market research project, including detailed reports on market trends, segment analysis, competitive landscape, regional insights, and forecasts, providing actionable intelligence for strategic decision-making.
What are the market trends of spinal Muscular Atrophy Treatment?
Current market trends in spinal muscular atrophy treatment include a focus on gene therapy solutions, increasing prevalence of SMA diagnoses, integration of digital health technologies, and a shift towards personalized care approaches driven by patient outcomes.