Spinal Non Fusion Devices Market Report
Published Date: 31 January 2026 | Report Code: spinal-non-fusion-devices
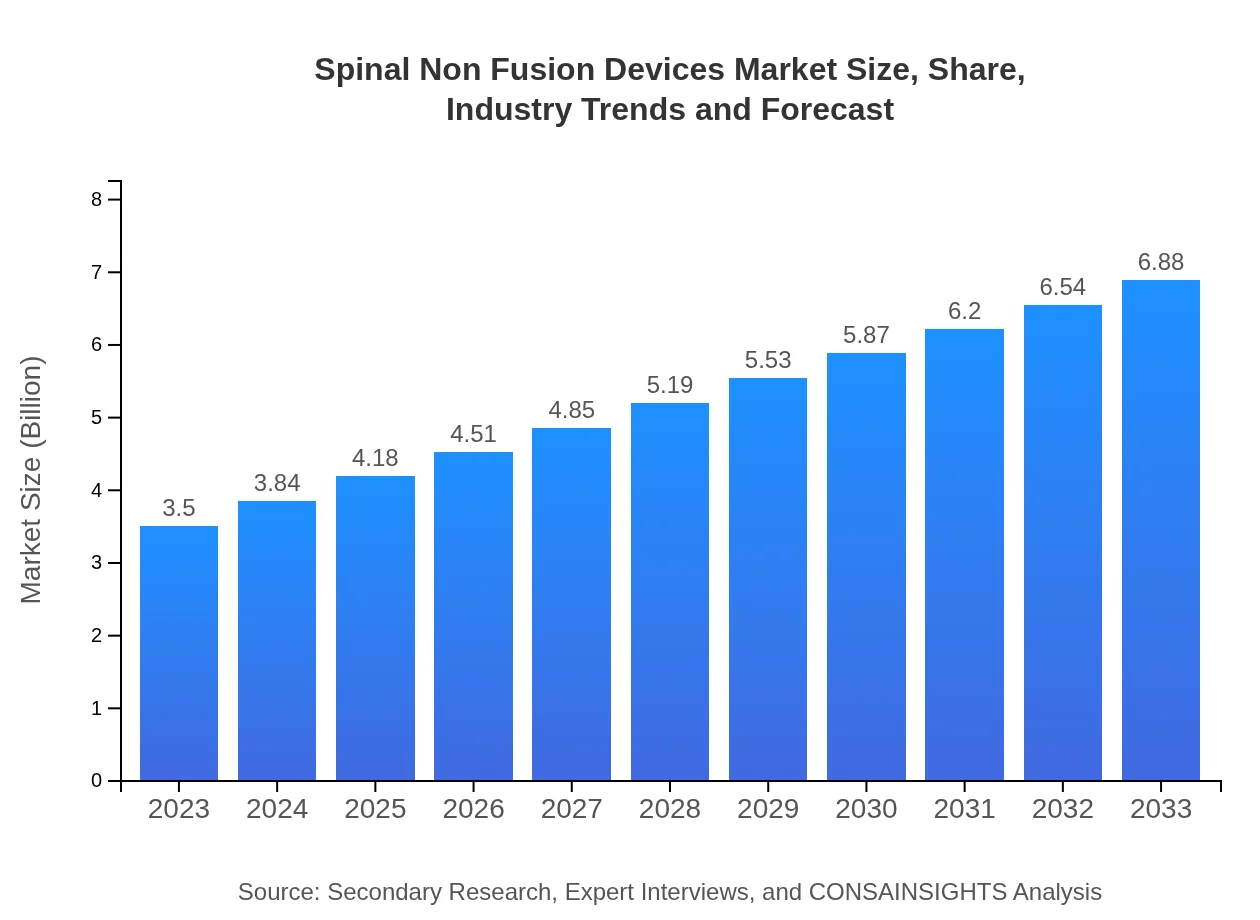
Spinal Non Fusion Devices Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Spinal Non Fusion Devices market, including current trends, market size forecasts, segmentation, and key players from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $3.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $6.88 Billion |
| Top Companies | Medtronic , Stryker Corporation, Johnson & Johnson, NuVasive, Globus Medical |
| Last Modified Date | 31 January 2026 |
Spinal Non Fusion Devices Market Overview
Customize Spinal Non Fusion Devices Market Report market research report
- ✔ Get in-depth analysis of Spinal Non Fusion Devices market size, growth, and forecasts.
- ✔ Understand Spinal Non Fusion Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Spinal Non Fusion Devices
What is the Market Size & CAGR of Spinal Non Fusion Devices market in 2023?
Spinal Non Fusion Devices Industry Analysis
Spinal Non Fusion Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Spinal Non Fusion Devices Market Analysis Report by Region
Europe Spinal Non Fusion Devices Market Report:
The European market for Spinal Non Fusion Devices is expected to witness steady growth from USD 0.85 billion in 2023 to USD 1.68 billion by 2033. The demand for advanced solutions in spine care, coupled with the supportive regulatory environment, is fostering new product development. Countries like Germany, France, and the UK are leading the pace in this sector.Asia Pacific Spinal Non Fusion Devices Market Report:
In the Asia Pacific, the Spinal Non Fusion Devices market is on an upward trajectory, expected to reach USD 1.42 billion by 2033, from USD 0.72 billion in 2023. Factors such as the growing elderly population and advancements in surgical techniques are driving market expansion. The increasing prevalence of obesity and related spinal disorders is another significant driver for market growth in this region.North America Spinal Non Fusion Devices Market Report:
North America dominates the Spinal Non Fusion Devices market with a valuation expanding from USD 1.28 billion in 2023 to USD 2.51 billion by 2033. Factors contributing to this robust growth include the rising geriatric demographics, highly developed healthcare systems, and a preference for minimally invasive surgeries. Additionally, key players are increasingly active in this region, focusing on extensive R&D.South America Spinal Non Fusion Devices Market Report:
The South American market for Spinal Non Fusion Devices is projected to grow from USD 0.25 billion in 2023 to USD 0.50 billion by 2033. This growth is prompted by rising healthcare investments, higher surgical volumes, and increased awareness of non-fusion treatment options. Brazil and Argentina remain the key markets, contributing to this growth trajectory.Middle East & Africa Spinal Non Fusion Devices Market Report:
In the Middle East and Africa, the market is expected to grow from USD 0.39 billion in 2023 to USD 0.77 billion by 2033. Growth drivers include a growing number of spine surgeries, rising healthcare infrastructure investments, and increased expenditure on advanced medical treatments. The region presents unique challenges owing to diverse healthcare access levels, suggesting an important area for development efforts.Tell us your focus area and get a customized research report.
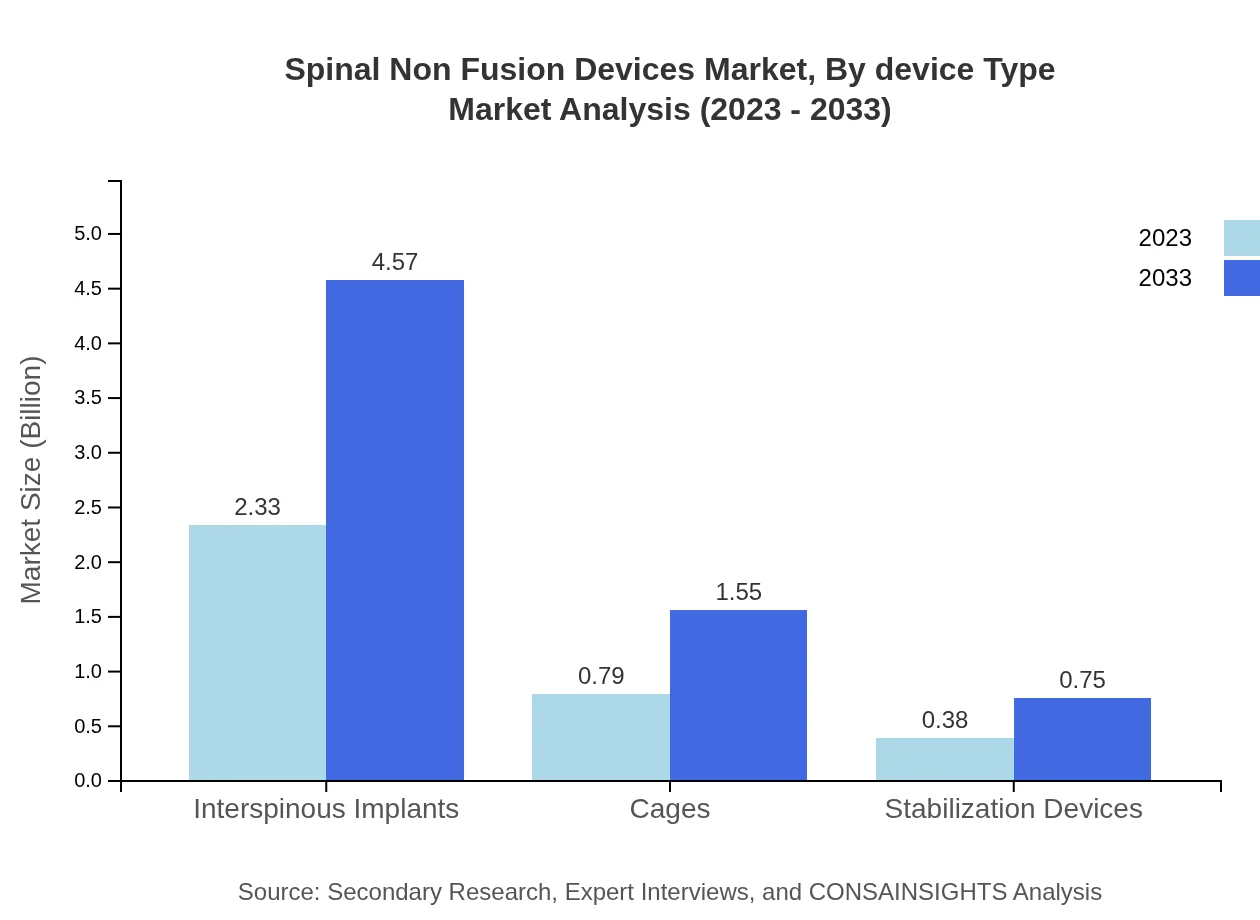
Spinal Non Fusion Devices Market Analysis By Device Type
The Spinal Non-Fusion Devices market by device type comprises interspinous implants, dynamic stabilization devices, and other innovative therapies. Interspinous implants, generating USD 2.33 billion in 2023, maintain 66.47% market share, while dynamic devices are rising in prominence due to their patient-friendly approach. Traditional technologies currently dominate, but advanced technologies are expected to see increasing adoption thanks to favorable outcomes.
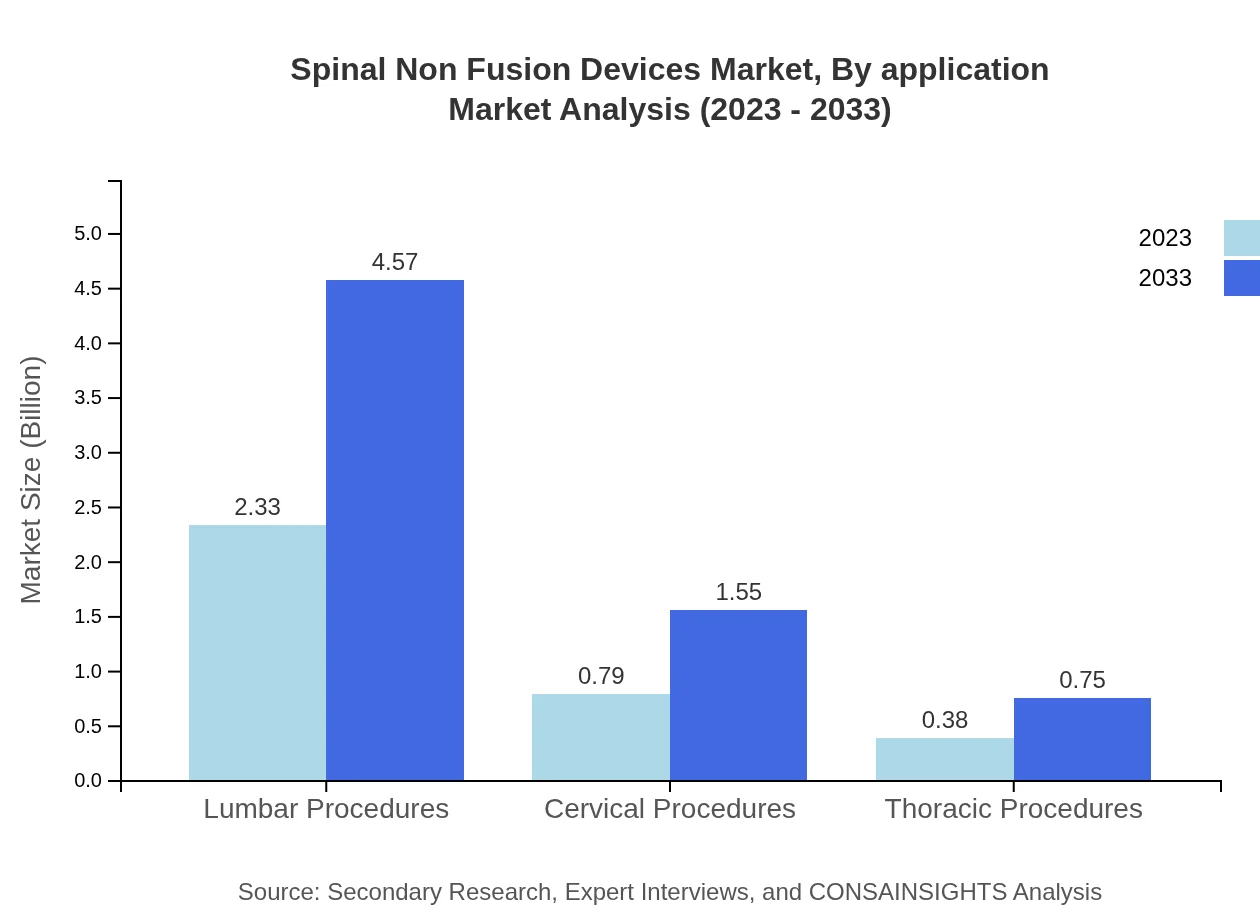
Spinal Non Fusion Devices Market Analysis By Application
Key applications in the Spinal Non-Fusion Devices market include lumbar, cervical, and thoracic procedures. The lumbar segment holds the largest market share due to the high incidence of lumbar degenerative diseases. The cervical segment is also rapidly growing, driven by rising cervical disc disease incidence and procedure preferences.
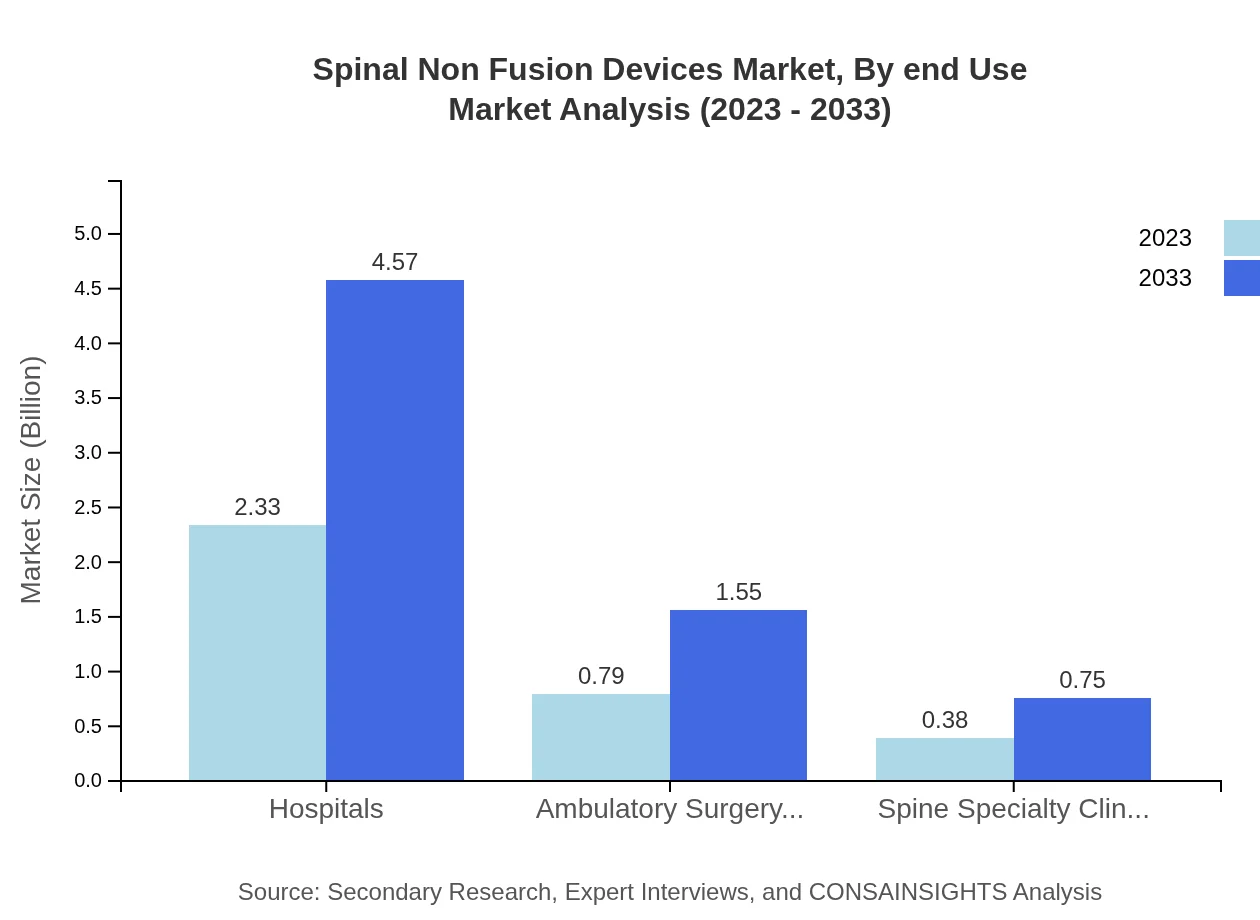
Spinal Non Fusion Devices Market Analysis By End Use
End-use segments include hospitals, ambulatory surgery centers, and spine specialty clinics. Hospitals are leading in market share, accounting for approximately 66.47% in 2023, driven by the availability of complex surgical facilities. Ambulatory surgery centers are gaining ground due to a shift in surgical preferences toward outpatient care.
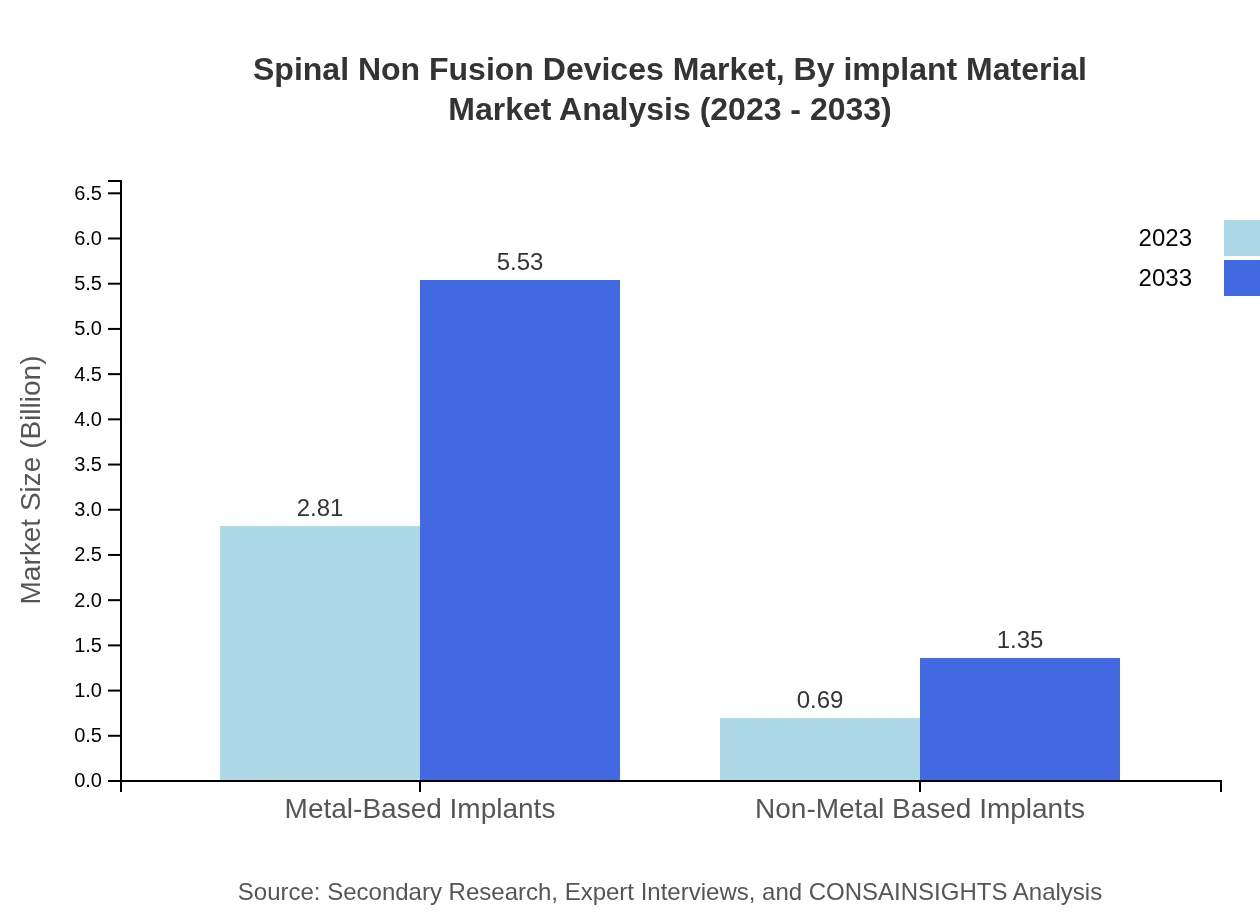
Spinal Non Fusion Devices Market Analysis By Implant Material
Implant materials in the market can be classified into metal-based and non-metal based implants. Metal-based implants continue to dominate the market thanks to their strength and reliability, accounting for 80.41% share in 2023. Non-metal based implants are growing due to increasing focus on biocompatibility and lesser complication rates.
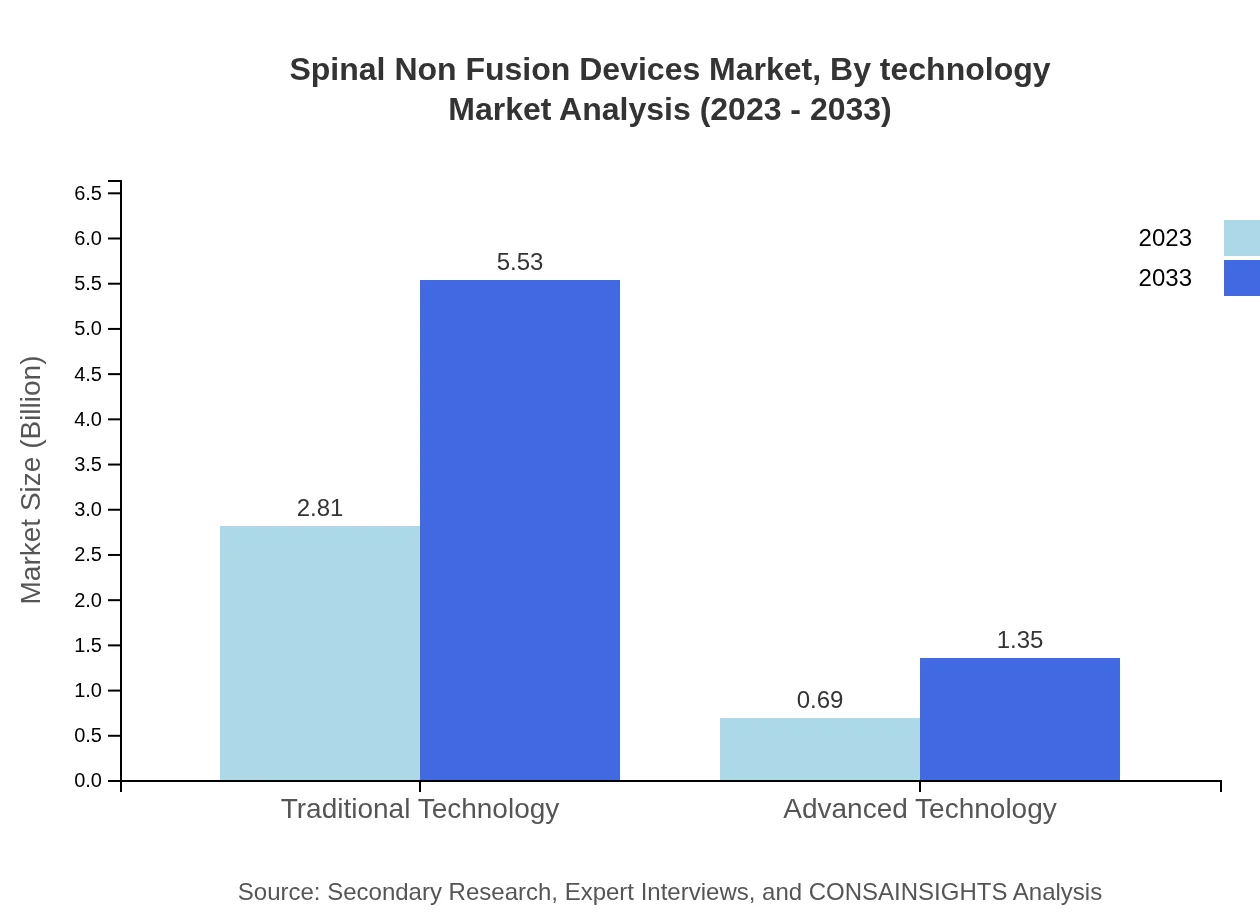
Spinal Non Fusion Devices Market Analysis By Technology
The Spinal Non-Fusion Devices market sees a differentiation between traditional and advanced technologies. Traditional technologies currently hold an 80.41% share. However, advanced technologies are rapidly being incorporated due to shifts towards less invasive surgical options, enhancing the overall growth potential of the market.
Spinal Non Fusion Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Spinal Non Fusion Devices Industry
Medtronic :
Medtronic is a global leader in medical technology, offering innovative spinal devices aimed at improving patient outcomes and advancing minimally invasive surgical techniques.Stryker Corporation:
Stryker is known for its wide-ranging portfolio of orthopedic products, including advanced spinal devices that facilitate outpatient care and enhance surgery recovery times.Johnson & Johnson:
With its broad healthcare offering, Johnson & Johnson manufactures specialized spinal non-fusion solutions that focus on biocompatibility and effective treatment outcomes.NuVasive:
NuVasive is a pioneer in spine surgery technology, renowned for its innovative non-fusion device solutions that support improved surgical precision and patient safety.Globus Medical:
Globus Medical focuses on developing technology that drives less invasive surgical procedures, offering a successful range of spinal non-fusion devices that enhance recovery.We're grateful to work with incredible clients.









FAQs
What is the market size of spinal non Fusion devices?
The spinal non-fusion devices market is valued at approximately $3.5 billion in 2023 and is expected to grow at a CAGR of 6.8% over the next decade. By 2033, the market is projected to expand significantly, reflecting rising demand.
What are the key market players or companies in this spinal non Fusion devices industry?
Key players in the spinal non-fusion devices market include Medtronic, DePuy Synthes (Johnson & Johnson), NuVasive, Stryker, and Globus Medical. These companies are known for their innovative products and strong market positions, contributing to overall industry growth.
What are the primary factors driving the growth in the spinal non Fusion devices industry?
The growth in the spinal non-fusion devices market is driven by increased aging population, rising prevalence of spine disorders, technological advancements, and a shift towards minimally invasive procedures that enhance surgical outcomes and patient recovery.
Which region is the fastest Growing in the spinal non Fusion devices?
The fastest-growing region in the spinal non-fusion devices market is projected to be Asia-Pacific. In 2023, the market value is $0.72 billion and is expected to reach $1.42 billion by 2033, reflecting a significant growth trajectory.
Does ConsaInsights provide customized market report data for the spinal non Fusion devices industry?
Yes, ConsaInsights offers customized market report data tailored for the spinal non-fusion devices industry. Clients can request specific analysis and insights based on unique business needs and market conditions.
What deliverables can I expect from this spinal non Fusion devices market research project?
Expect comprehensive deliverables including market analysis, growth forecasts, competitive landscape insights, regional breakdowns, and strategic recommendations. Reports will be tailored to meet specific client objectives.
What are the market trends of spinal non Fusion devices?
Market trends include an increasing preference for outpatient surgeries, advancements in implant technologies, a rise in interspinous implants, and a focus on non-metal-based implants. These trends highlight ongoing innovations and shifting patient desires.