Spinal Trauma Devices Market Report
Published Date: 31 January 2026 | Report Code: spinal-trauma-devices
Spinal Trauma Devices Market Size, Share, Industry Trends and Forecast to 2033
This report examines the Spinal Trauma Devices market, providing insights into trends, growth rates, and segmentation across regions from 2023 to 2033. It explores the market dynamics, key players, and forecasts that inform stakeholders in making strategic decisions.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
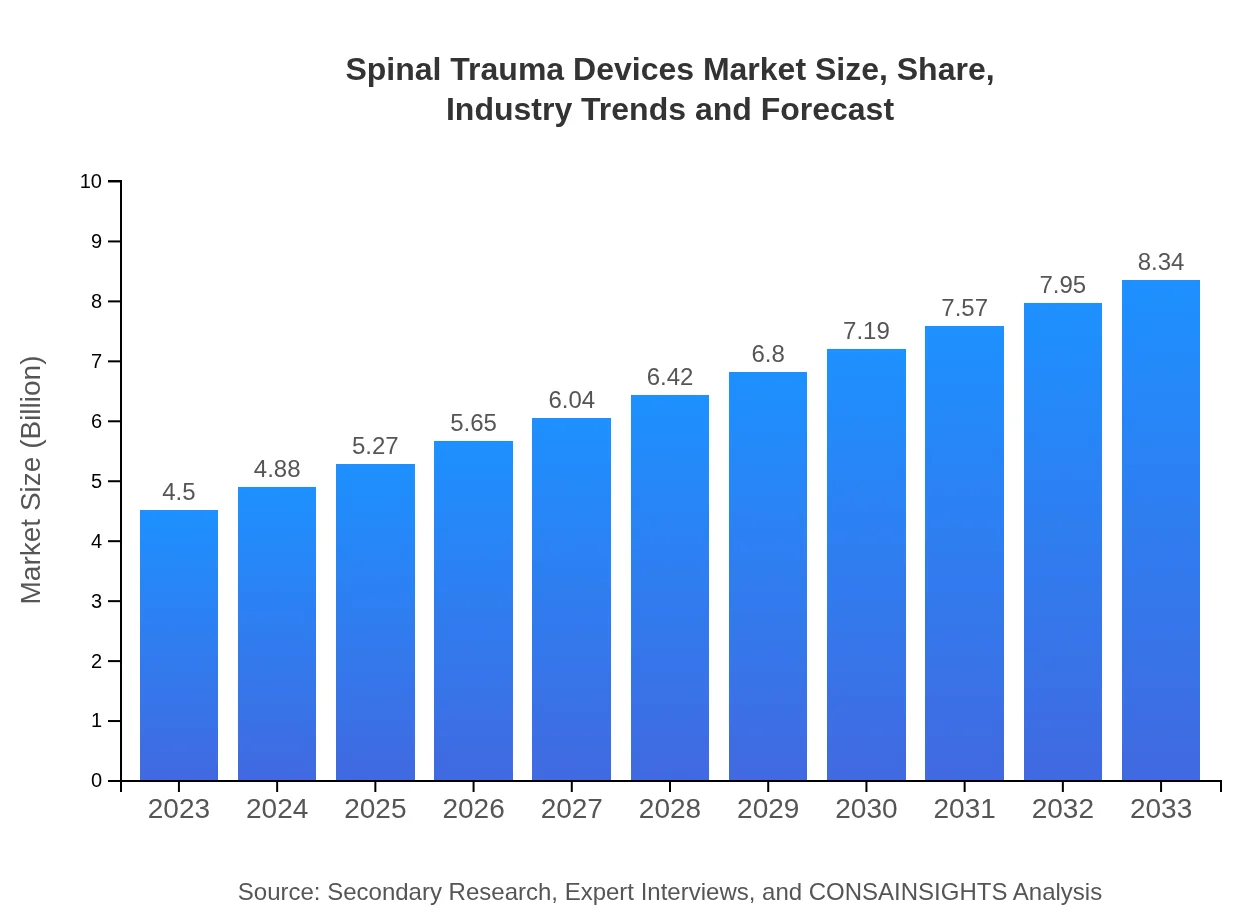
| 2023 Market Size | $4.50 Billion |
| CAGR (2023-2033) | 6.2% |
| 2033 Market Size | $8.34 Billion |
| Top Companies | Medtronic , Johnson & Johnson, Stryker , NuVasive, Zimmer Biomet |
| Last Modified Date | 31 January 2026 |
Spinal Trauma Devices Market Overview
Customize Spinal Trauma Devices Market Report market research report
- ✔ Get in-depth analysis of Spinal Trauma Devices market size, growth, and forecasts.
- ✔ Understand Spinal Trauma Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Spinal Trauma Devices
What is the Market Size & CAGR of Spinal Trauma Devices market in 2023?
Spinal Trauma Devices Industry Analysis
Spinal Trauma Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Spinal Trauma Devices Market Analysis Report by Region
Europe Spinal Trauma Devices Market Report:
Europe's market will expand from $1.38 billion in 2023 to $2.56 billion by 2033. Innovative healthcare solutions, strong regulatory frameworks, and an aging population are propelling demand for spinal trauma devices. Countries like Germany and France are significant contributors to this growth.Asia Pacific Spinal Trauma Devices Market Report:
In the Asia Pacific region, the Spinal Trauma Devices market is expected to grow from $0.92 billion in 2023 to $1.71 billion by 2033. The growth is attributed to rising healthcare investments, a large patient population, and increasing awareness regarding spinal health. Countries like China and India are leading the market due to their extensive healthcare reforms.North America Spinal Trauma Devices Market Report:
North America's Spinal Trauma Devices market is anticipated to increase from $1.46 billion in 2023 to $2.70 billion by 2033. The United States holds a dominant share, driven by technological advancements, widespread awareness of spinal health, and a robust healthcare system supporting patient needs.South America Spinal Trauma Devices Market Report:
The South American market is expected to grow from $0.23 billion in 2023 to $0.42 billion by 2033. Factors such as increasing healthcare spending and a rise in road accidents are key contributors. However, challenges such as economic instability and healthcare accessibility remain.Middle East & Africa Spinal Trauma Devices Market Report:
The Middle East and Africa market will grow from $0.52 billion in 2023 to $0.96 billion by 2033. The increasing number of spine surgeries and rising regional healthcare investment illustrate substantial growth potential, despite challenges related to healthcare infrastructure in many countries.Tell us your focus area and get a customized research report.
Spinal Trauma Devices Market Analysis By Product
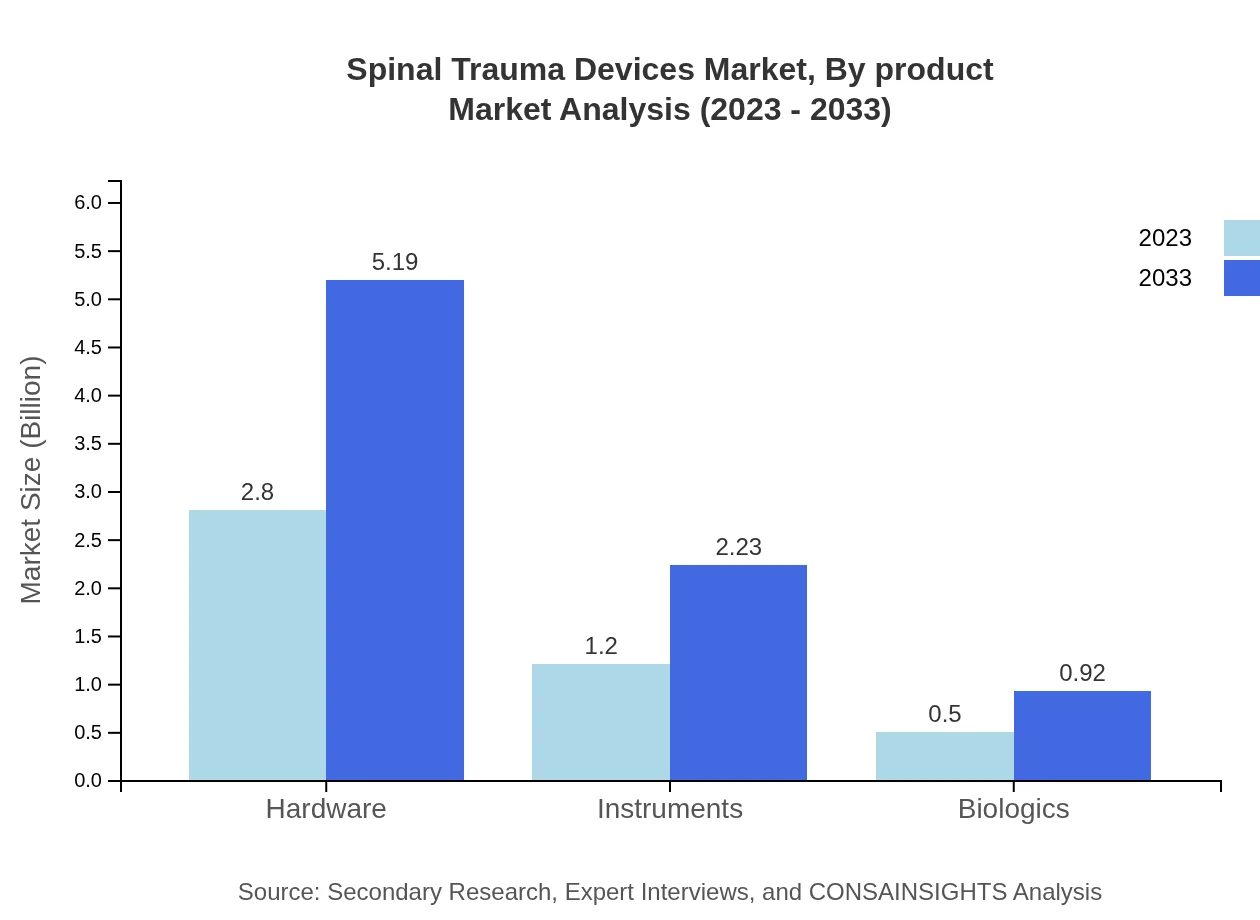
The product segmentation reveals that hardware dominates the market, with sales expected to rise from $2.80 billion in 2023 to $5.19 billion by 2033, accounting for a 62.25% market share. Instruments and biologics also represent significant segments, with the instruments market projected to grow from $1.20 billion to $2.23 billion, holding a 26.73% share. Biologics are expected to increase from $0.50 billion to $0.92 billion, gaining an 11.02% market share, showcasing an increased preference for biological solutions in spinal injury treatment.
Spinal Trauma Devices Market Analysis By Application
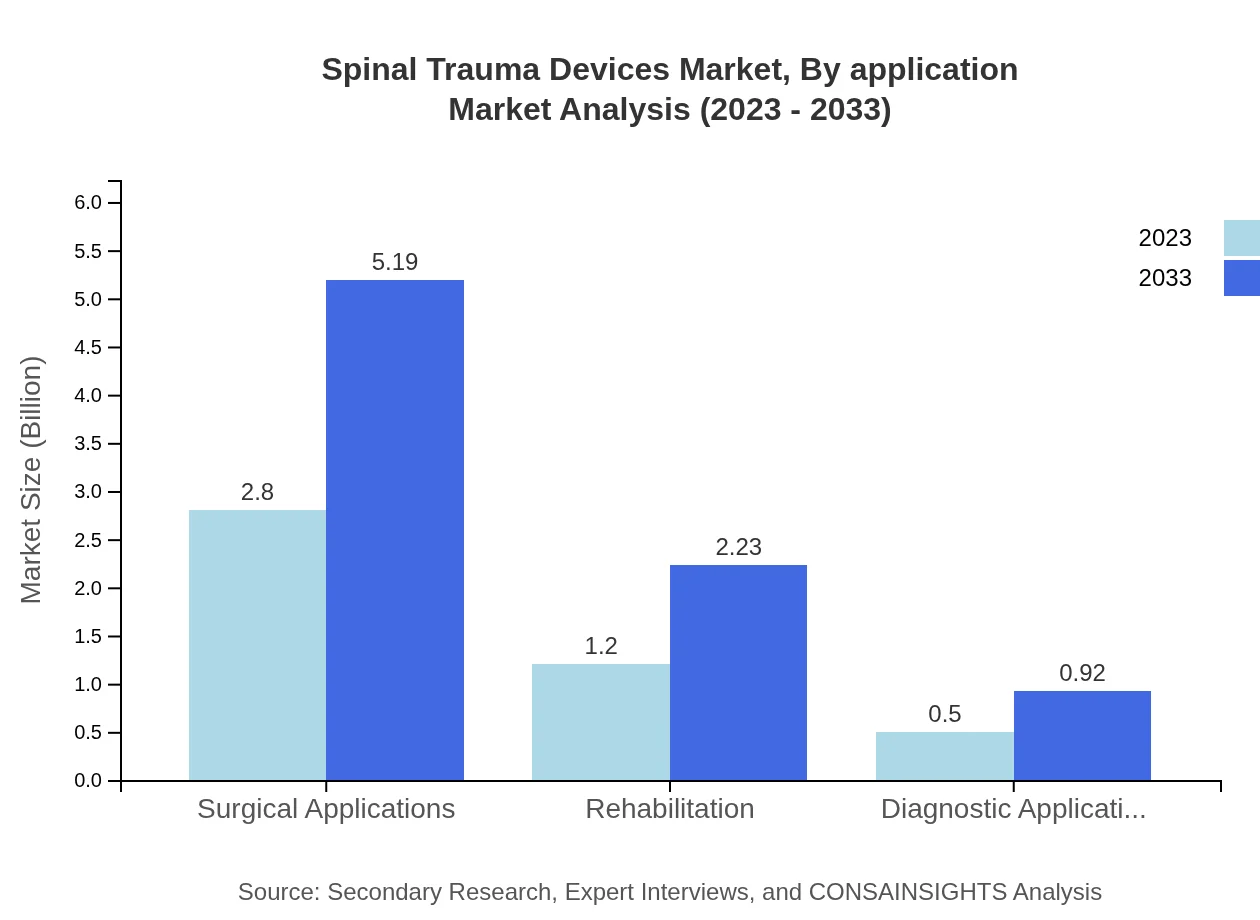
The application segments include surgical and non-surgical treatments, with surgical procedures holding a significant market share of 80.81% combined by growing from $3.64 billion to $6.74 billion. Non-surgical treatments are also trending upwards, with growth from $0.86 billion to $1.60 billion. This shift reflects advancements in non-invasive technologies and alternative treatment strategies gaining traction.
Spinal Trauma Devices Market Analysis By End User
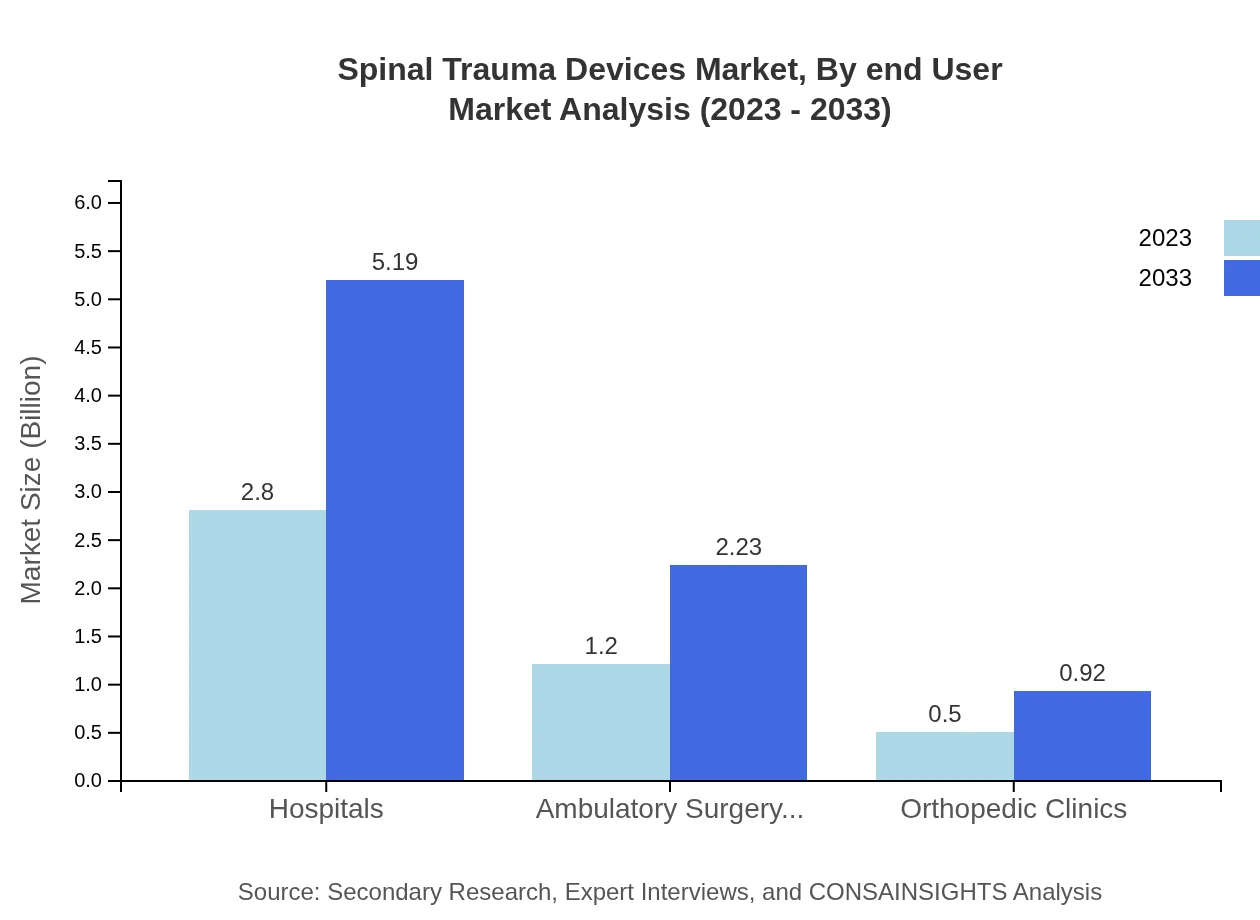
Hospitals account for the largest share of the market at 62.25%, with projected growth from $2.80 billion in 2023 to $5.19 billion by 2033. Ambulatory Surgery Centers will also show growth from $1.20 billion to $2.23 billion, maintaining a 26.73% market share. Orthopedic Clinics at 11.02% demonstrate the smaller, yet vital, role these specialized centers play in spinal trauma care.
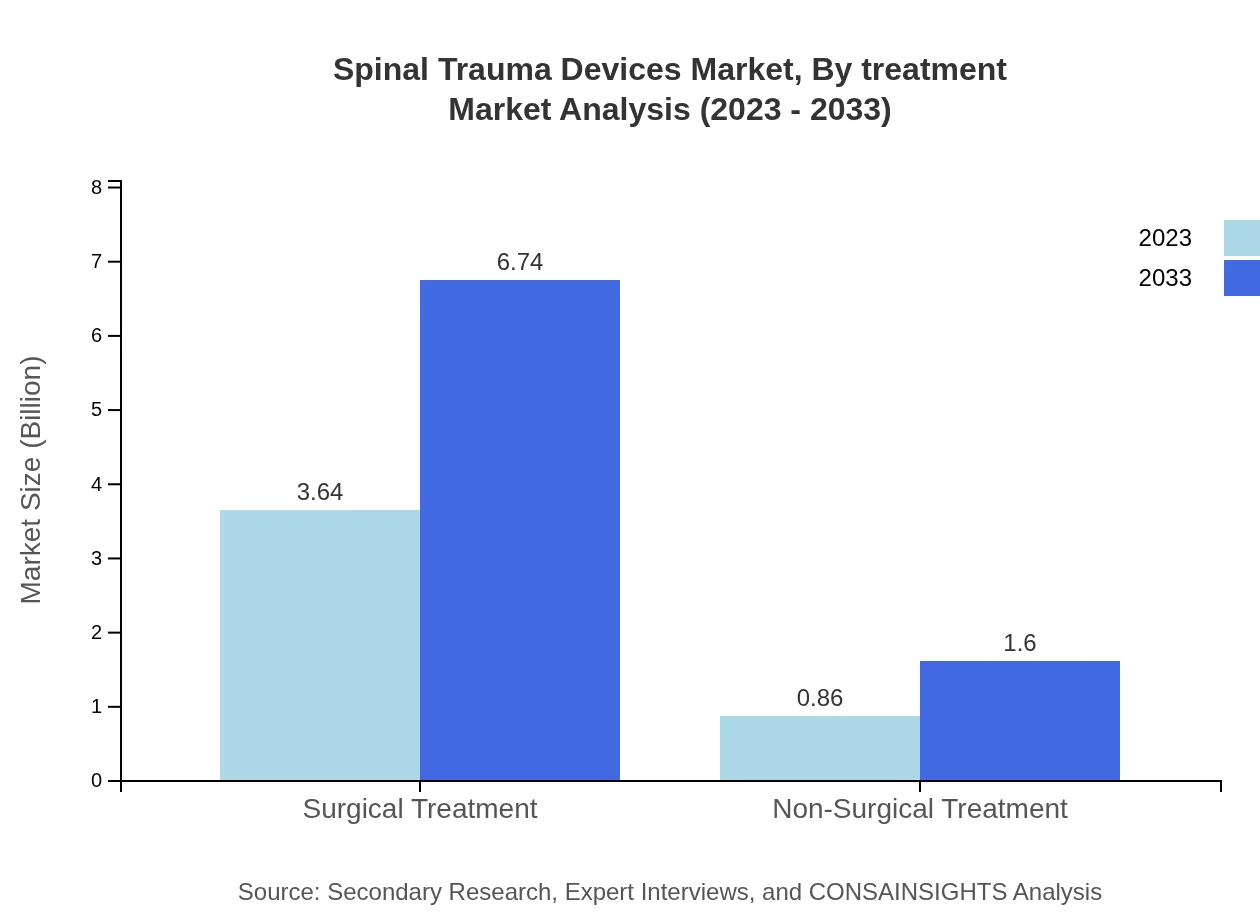
Spinal Trauma Devices Market Analysis By Treatment
Surgical treatments dominate the market, expected to grow from $3.64 billion to $6.74 billion. Non-surgical treatments, while smaller, are also growing from $0.86 billion to $1.60 billion. This data indicates a trend towards more significant investment in treatment options that improve both treatment efficacy and patient recovery time.
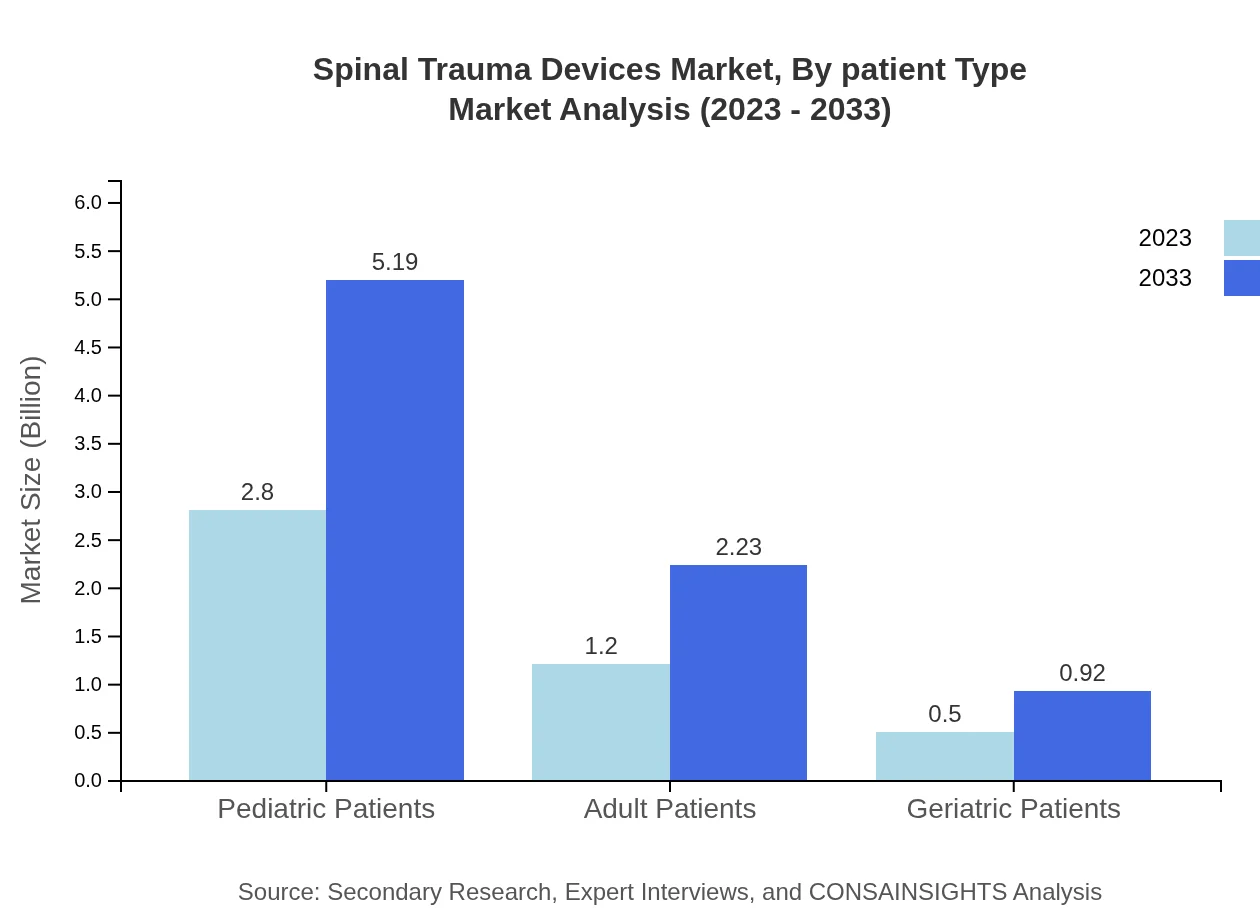
Spinal Trauma Devices Market Analysis By Patient Type
The market is segmented by patient type, with pediatric patients currently holding a 62.25% share growing from $2.80 billion to $5.19 billion. Adult patients are projected to grow from $1.20 billion to $2.23 billion, capturing 26.73% of the market, whereas geriatric patients, contributing 11.02%, are anticipated to rise from $0.50 billion to $0.92 billion as aging populations increase globally.
Spinal Trauma Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Spinal Trauma Devices Industry
Medtronic :
A leading innovator in spinal care, Medtronic offers a wide array of spinal devices and surgical instruments recognized for their quality and effectiveness.Johnson & Johnson:
Through its DePuy Synthes division, Johnson & Johnson develops and provides spinal injury products that are trusted by many healthcare professionals globally.Stryker :
Stryker specializes in orthopedic products, including spinal devices that emphasize innovation and enhanced patient outcomes.NuVasive:
NuVasive focuses on minimally invasive spine surgery and offers cutting-edge technological solutions in spinal trauma treatment.Zimmer Biomet:
Zimmer Biomet is known for its advanced spinal products, contributing to surgical and rehabilitation phases of spinal trauma care.We're grateful to work with incredible clients.









FAQs
What is the market size of spinal Trauma Devices?
As of 2023, the spinal trauma devices market is valued at approximately $4.5 billion, with a projected CAGR of 6.2% through 2033. This growth signifies an expanding landscape for advancements in spinal treatment.
What are the key market players or companies in the spinal trauma devices industry?
The spinal trauma devices industry is characterized by notable players including Medtronic, DePuy Synthes, NuVasive, and Stryker. These companies lead the market, offering innovative products and expanding their portfolios to meet growing orthopedic demands.
What are the primary factors driving the growth in the spinal trauma devices industry?
Key growth drivers include increasing incidences of spinal injuries, an aging population, technological advancements in surgical procedures, and the rising demand for minimally invasive surgeries. These factors fuel the need for enhanced spinal trauma devices.
Which region is the fastest Growing in the spinal trauma devices market?
The North America region is currently the fastest-growing market for spinal trauma devices. The market is projected to grow from $1.46 billion in 2023 to $2.70 billion by 2033, benefiting from advanced healthcare infrastructure and rising surgical prevalence.
Does ConsaInsights provide customized market report data for the spinal trauma devices industry?
Yes, ConsaInsights specializes in providing customized market reports tailored to specific client needs within the spinal trauma devices market. This customization allows stakeholders to focus on relevant data and insights for strategic decision-making.
What deliverables can I expect from this spinal trauma devices market research project?
Expect comprehensive deliverables including detailed market analysis, segment insights, competitive landscape evaluations, regional breakdowns, and future projections. Data will be presented in an accessible format, aiding in effective strategic planning.
What are the market trends of spinal trauma devices?
Current trends in the spinal trauma devices sector include a shift towards minimally invasive surgical techniques, increased adoption of robotics in surgery, and innovations in biologics and hardware solutions, indicating a dynamic evolution in patient care.