Stem Cell Manufacturing Market Report
Published Date: 31 January 2026 | Report Code: stem-cell-manufacturing
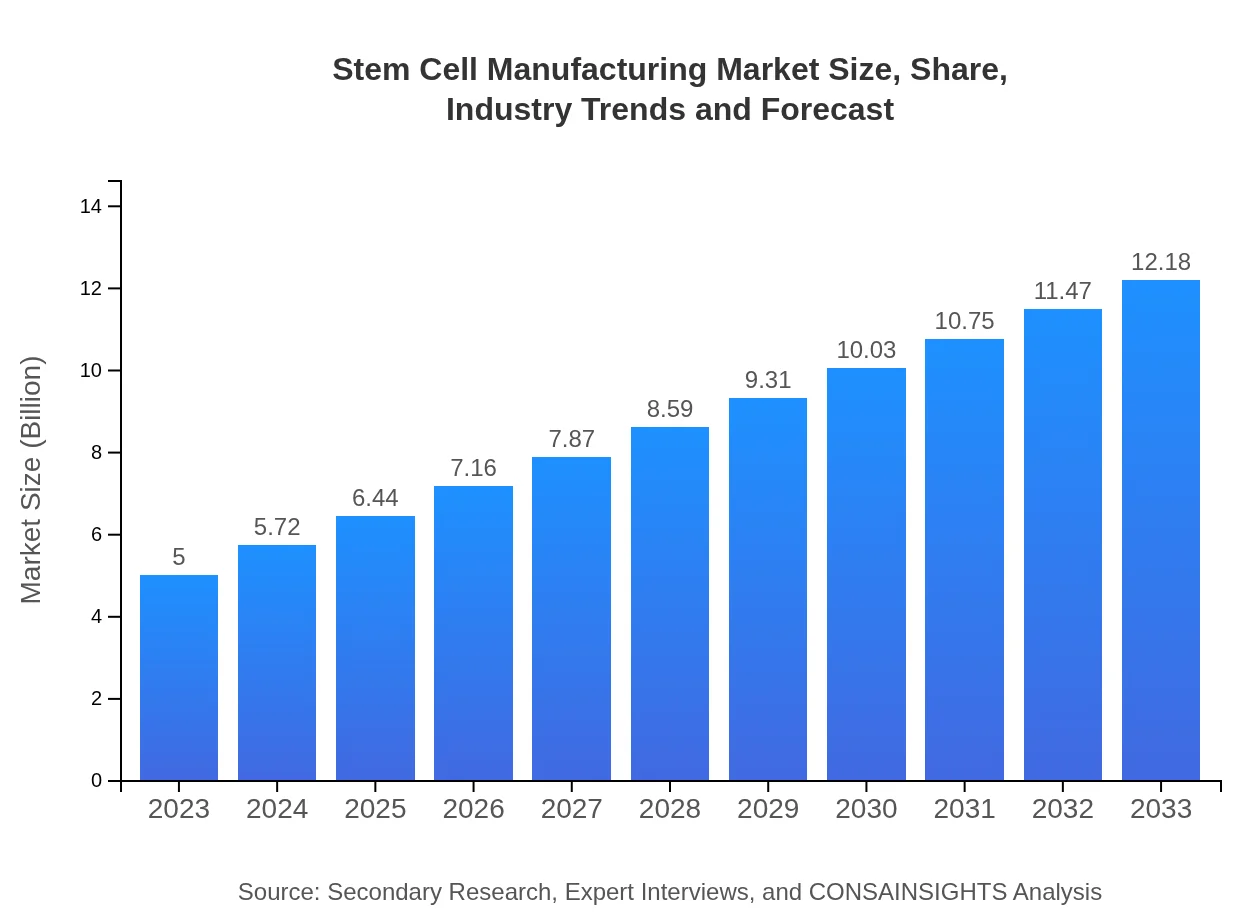
Stem Cell Manufacturing Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the stem cell manufacturing market, focusing on market size, trends, forecasts, and segmentation from 2023 to 2033. Key insights on regional performance, technology advancements, and leading companies are also included.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $5.00 Billion |
| CAGR (2023-2033) | 9% |
| 2033 Market Size | $12.18 Billion |
| Top Companies | Thermo Fisher Scientific, Lonza Group AG, Merck KGaA, STEMCELL Technologies |
| Last Modified Date | 31 January 2026 |
Stem Cell Manufacturing Market Overview
Customize Stem Cell Manufacturing Market Report market research report
- ✔ Get in-depth analysis of Stem Cell Manufacturing market size, growth, and forecasts.
- ✔ Understand Stem Cell Manufacturing's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Stem Cell Manufacturing
What is the Market Size & CAGR of Stem Cell Manufacturing market in 2023?
Stem Cell Manufacturing Industry Analysis
Stem Cell Manufacturing Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Stem Cell Manufacturing Market Analysis Report by Region
Europe Stem Cell Manufacturing Market Report:
In Europe, the market size will expand from $1.46 billion in 2023 to $3.57 billion by 2033. This growth represents a CAGR of 9.3%, as the region focuses on advanced cell therapies and innovative manufacturing technologies.Asia Pacific Stem Cell Manufacturing Market Report:
In 2023, the Asia Pacific stem cell manufacturing market is valued at $0.89 billion, projected to grow to $2.17 billion by 2033, representing a strong CAGR of 9.5%. This region is characterized by increasing investments in biopharmaceuticals and regenerative medicine, with countries such as Japan and China leading in research advancements.North America Stem Cell Manufacturing Market Report:
North America leads the market with a valuation of $1.92 billion in 2023, expected to reach $4.67 billion by 2033. The region exhibits a robust CAGR of 10.2% due to heightened R&D activities, a favorable regulatory environment, and the presence of leading pharmaceutical companies.South America Stem Cell Manufacturing Market Report:
The South American market is anticipated to grow from $0.44 billion in 2023 to $1.08 billion by 2033. The CAGR for this region is approximately 9.1%. Growing awareness of stem cell therapies and improvements in healthcare infrastructure are driving market growth.Middle East & Africa Stem Cell Manufacturing Market Report:
The market in the Middle East and Africa is projected to grow from $0.29 billion in 2023 to $0.70 billion by 2033, indicating a CAGR of 9.2%. Growing investment in healthcare and biology research, along with increasing awareness of stem cell therapies, are crucial growth factors.Tell us your focus area and get a customized research report.
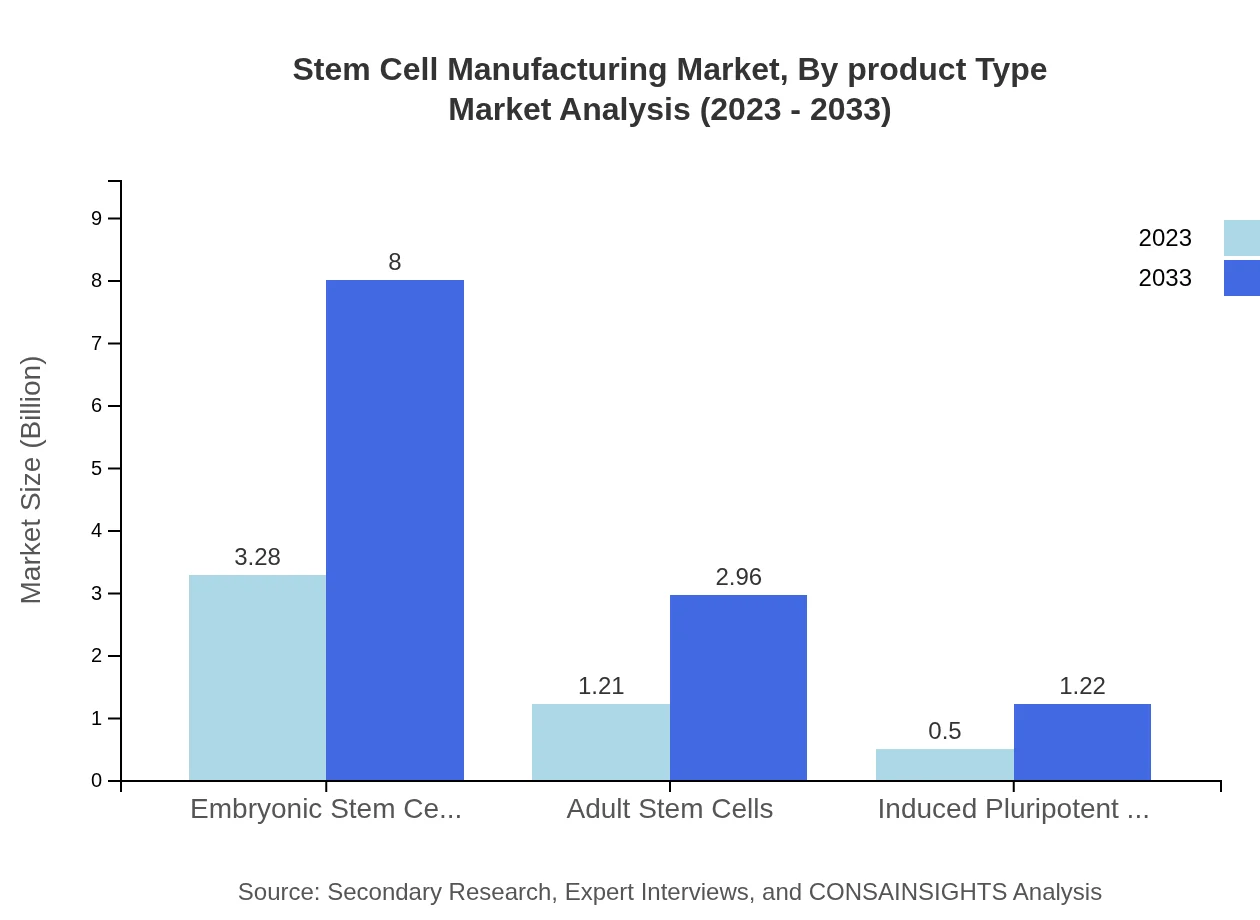
Stem Cell Manufacturing Market Analysis By Product Type
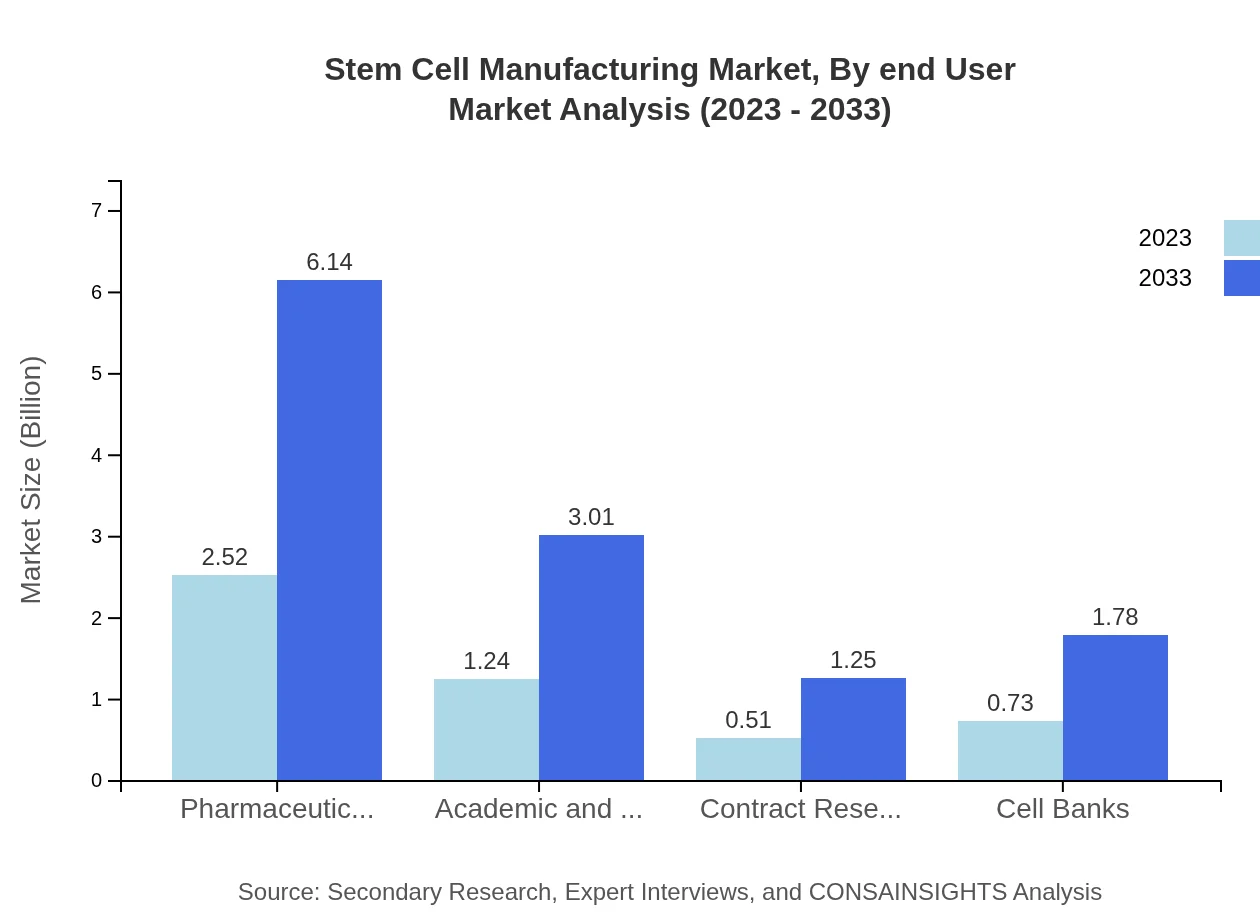
The pharmaceutical companies segment holds a significant share of the market, valued at $2.52 billion in 2023 and projected to increase to $6.14 billion by 2033. Academic and research institutions are also notable, with a current market size of $1.24 billion expected to grow to $3.01 billion. Additionally, Contract Research Organizations (CROs), currently worth $0.51 billion, and Cell Banks, with a market value of $0.73 billion, will also experience growth over this period.
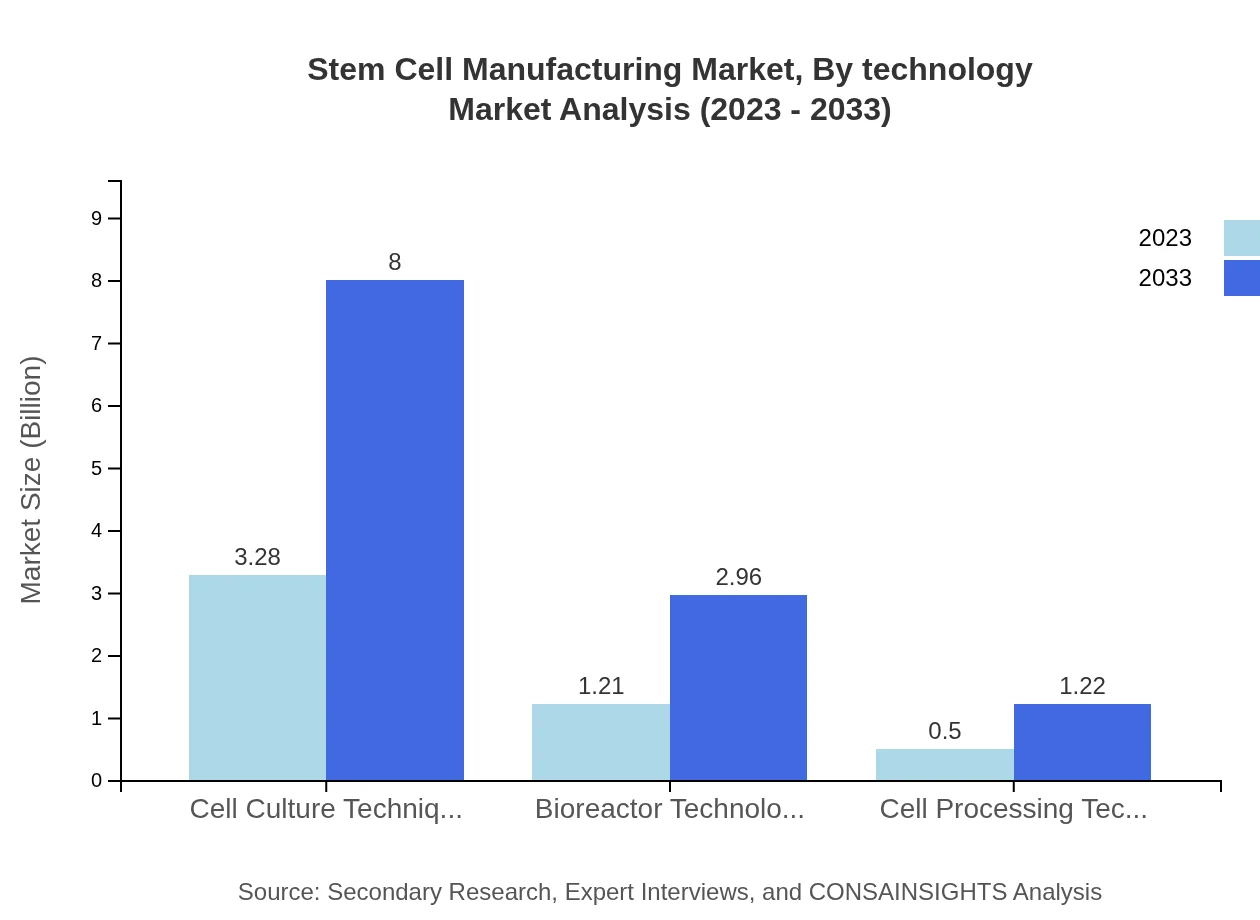
Stem Cell Manufacturing Market Analysis By Technology
Cell culture techniques dominate the market, accounting for 65.65% market share in 2023, valued at $3.28 billion and expected to reach $8.00 billion by 2033. Bioreactor technologies follow, with a market value of $1.21 billion in 2023, projected to grow to $2.96 billion. Other relevant technologies include cell processing technologies, valued at $0.50 billion in 2023, forecasted to reach $1.22 billion by 2033.
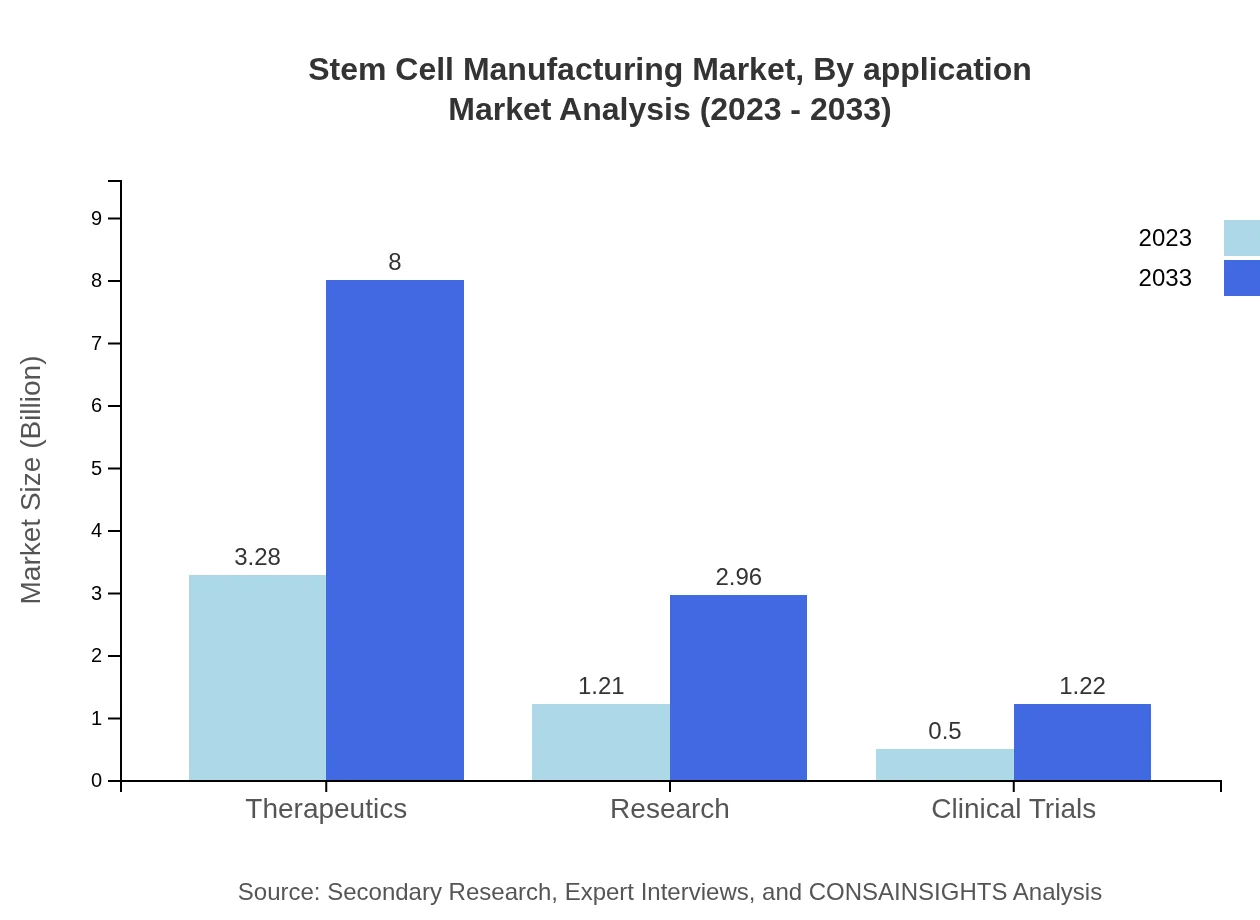
Stem Cell Manufacturing Market Analysis By Application
Therapeutics represent the largest segment of the market, with a current size of $3.28 billion and a dominance of 65.65% share, expected to grow to $8.00 billion by 2033. Moreover, research applications are valued at $1.21 billion in 2023, with anticipated growth to $2.96 billion, while clinical trials account for $0.50 billion in the current year, expected to expand to $1.22 billion.
Stem Cell Manufacturing Market Analysis By End User
Hospitals and clinics are the primary end-users, driving the demand for stem cell manufacturing as they increasingly adopt advanced therapies. This segment, along with research laboratories, represents a significant market size, supported by rising patient awareness and the integration of innovative therapies into clinical practices.
Stem Cell Manufacturing Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Stem Cell Manufacturing Industry
Thermo Fisher Scientific:
A leading provider of life science solutions, Thermo Fisher Scientific specializes in high-quality stem cell culture systems and cell processing technologies, enhancing research and therapeutic applications.Lonza Group AG:
Lonza is a global leader in biologics manufacturing and a pioneer in stem cell technologies, offering essential services for the commercialization of cell and gene therapies.Merck KGaA:
Merck KGaA offers a broad portfolio of products for stem cell research, focusing on advanced cell culture techniques and bioprocessing technologies to drive innovation in the regenerative medicine space.STEMCELL Technologies:
STEMCELL Technologies is a biotechnology company that develops specialized culture media, tools, and technologies specifically for stem cell research and regenerative medicine applications.We're grateful to work with incredible clients.









FAQs
What is the market size of stem Cell Manufacturing?
The global stem cell manufacturing market is projected to reach $5 billion by 2033, with a compound annual growth rate (CAGR) of 9% from 2023. This growth reflects the increasing demand for stem cell-based therapies and products worldwide.
What are the key market players or companies in the stem Cell Manufacturing industry?
Key players in the stem cell manufacturing market include major pharmaceutical companies that develop stem cell therapies. These companies focus on innovation in regenerative medicine, which drives competitive dynamics within the industry.
What are the primary factors driving the growth in the stem Cell Manufacturing industry?
Growth drivers for the stem cell manufacturing industry include advancements in technology, increasing healthcare expenditure, and heightened awareness of regenerative medicine. Moreover, supportive government regulations and rising investments in stem cell research further stimulate market expansion.
Which region is the fastest Growing in the stem Cell Manufacturing market?
The fastest-growing region in the stem cell manufacturing market is North America, expected to grow from $1.92 billion in 2023 to $4.67 billion by 2033. This significant growth is driven by advancements in biotechnology and favorable regulatory frameworks.
Does ConsaInsights provide customized market report data for the stem Cell Manufacturing industry?
Yes, ConsaInsights offers customized market report data for the stem cell manufacturing industry. Clients can request tailored insights based on specific needs, allowing them to make informed decisions tailored to their strategic goals.
What deliverables can I expect from this stem Cell Manufacturing market research project?
Deliverables from the stem cell manufacturing market research project include detailed market analysis, segmentation insights, competitive landscape assessments, and forecasts. Clients will receive comprehensive reports to support strategic planning and decision-making.
What are the market trends of stem Cell Manufacturing?
Current market trends in stem cell manufacturing include increased investment in cell-based therapeutics, advances in bioreactor technologies, and a focus on personalized medicine. Collaborations between academia and industry are also driving innovation and expanding applications.