Stem Cell Therapy Market Report
Published Date: 31 January 2026 | Report Code: stem-cell-therapy
Stem Cell Therapy Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Stem Cell Therapy market, exploring industry insights, trends, regional performance, and forecasts from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
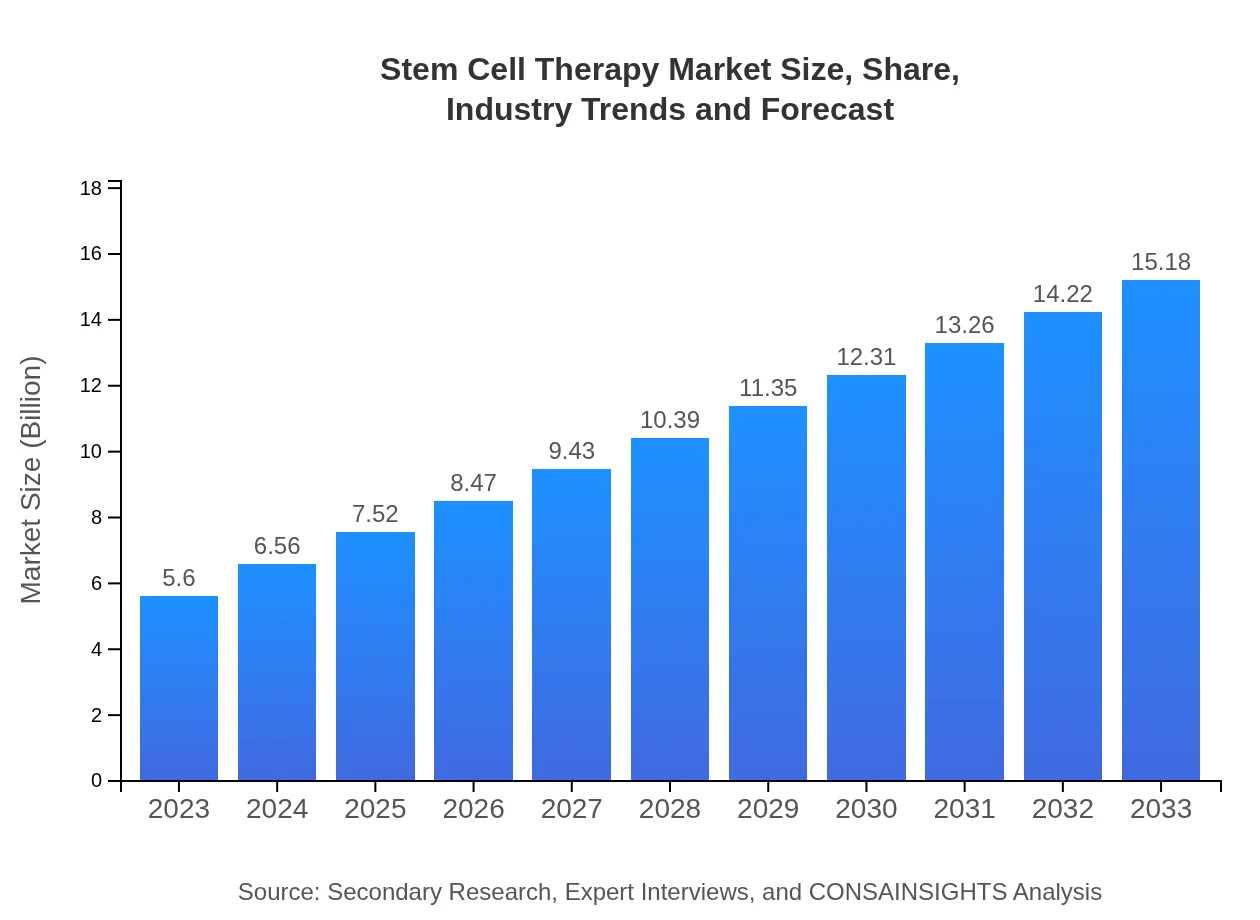
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 10.1% |
| 2033 Market Size | $15.18 Billion |
| Top Companies | Mesoblast Limited, Athersys, Inc., Osiris Therapeutics, Inc., StemCells, Inc. |
| Last Modified Date | 31 January 2026 |
Stem Cell Therapy Market Overview
Customize Stem Cell Therapy Market Report market research report
- ✔ Get in-depth analysis of Stem Cell Therapy market size, growth, and forecasts.
- ✔ Understand Stem Cell Therapy's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Stem Cell Therapy
What is the Market Size & CAGR of Stem Cell Therapy market in 2023?
Stem Cell Therapy Industry Analysis
Stem Cell Therapy Market Segmentation and Scope
Tell us your focus area and get a customized research report.
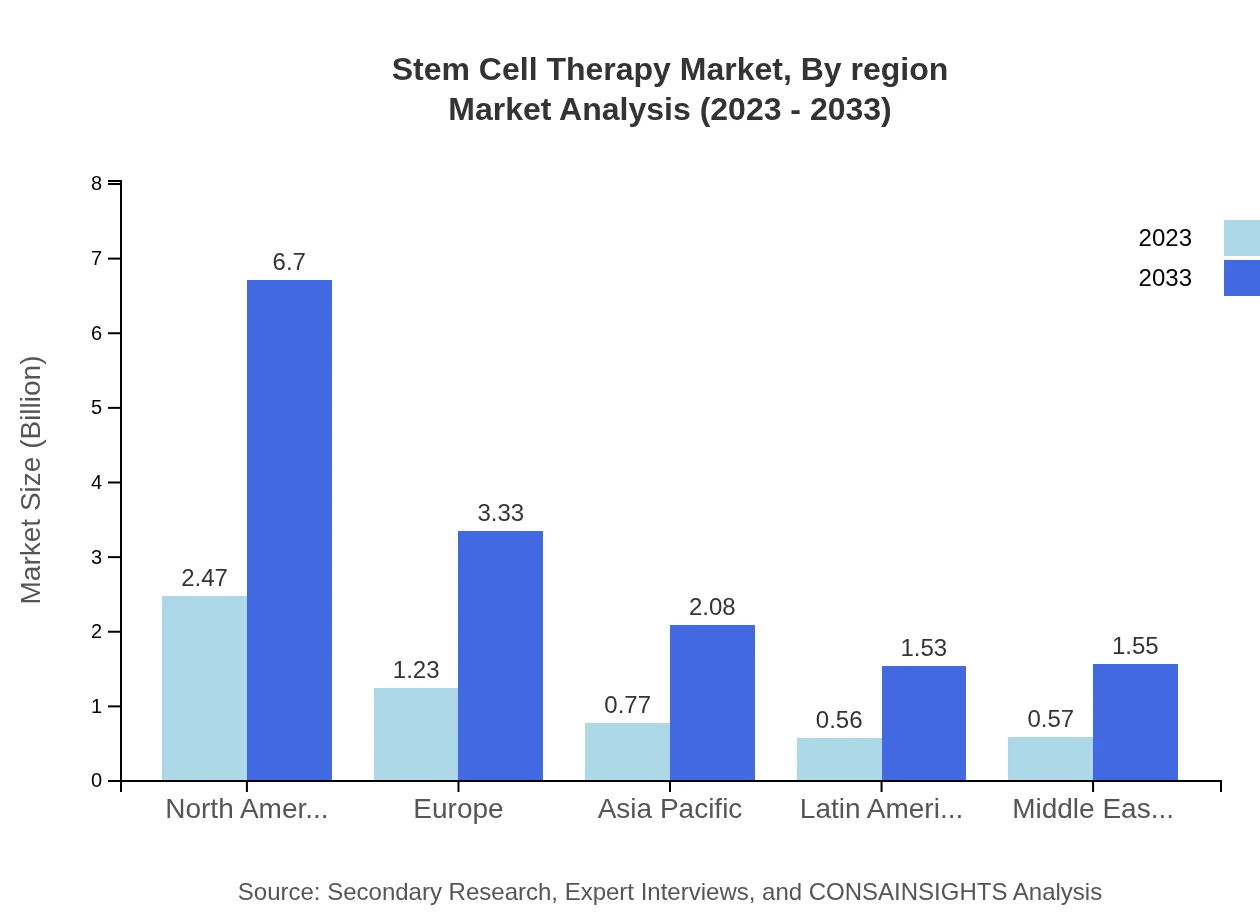
Stem Cell Therapy Market Analysis Report by Region
Europe Stem Cell Therapy Market Report:
The European market, sized at $1.58 billion in 2023, is projected to grow to $4.27 billion by 2033, supported by a strong emphasis on biomedical research and supportive government policies promoting regenerative medicine.Asia Pacific Stem Cell Therapy Market Report:
In 2023, the Asia Pacific market is valued at $1.06 billion, expected to expand to $2.87 billion by 2033, highlighting strong growth driven by increasing healthcare access and investments in biotechnological advancements.North America Stem Cell Therapy Market Report:
North America holds the largest market size of $2.14 billion in 2023, with an anticipated increase to $5.79 billion by 2033, primarily spurred by robust research communities and strong regulatory frameworks for stem cell applications.South America Stem Cell Therapy Market Report:
The South American Stem Cell Therapy market is comparatively smaller, estimated at $0.10 billion in 2023, with projections to reach $0.26 billion by 2033, reflecting growing interest in regenerative therapies amidst rising chronic disease prevalence.Middle East & Africa Stem Cell Therapy Market Report:
In the Middle East and Africa, the market stands at $0.73 billion in 2023 and is expected to grow to $1.98 billion by 2033, driven by improving healthcare infrastructure and increased awareness of stem cell therapies.Tell us your focus area and get a customized research report.
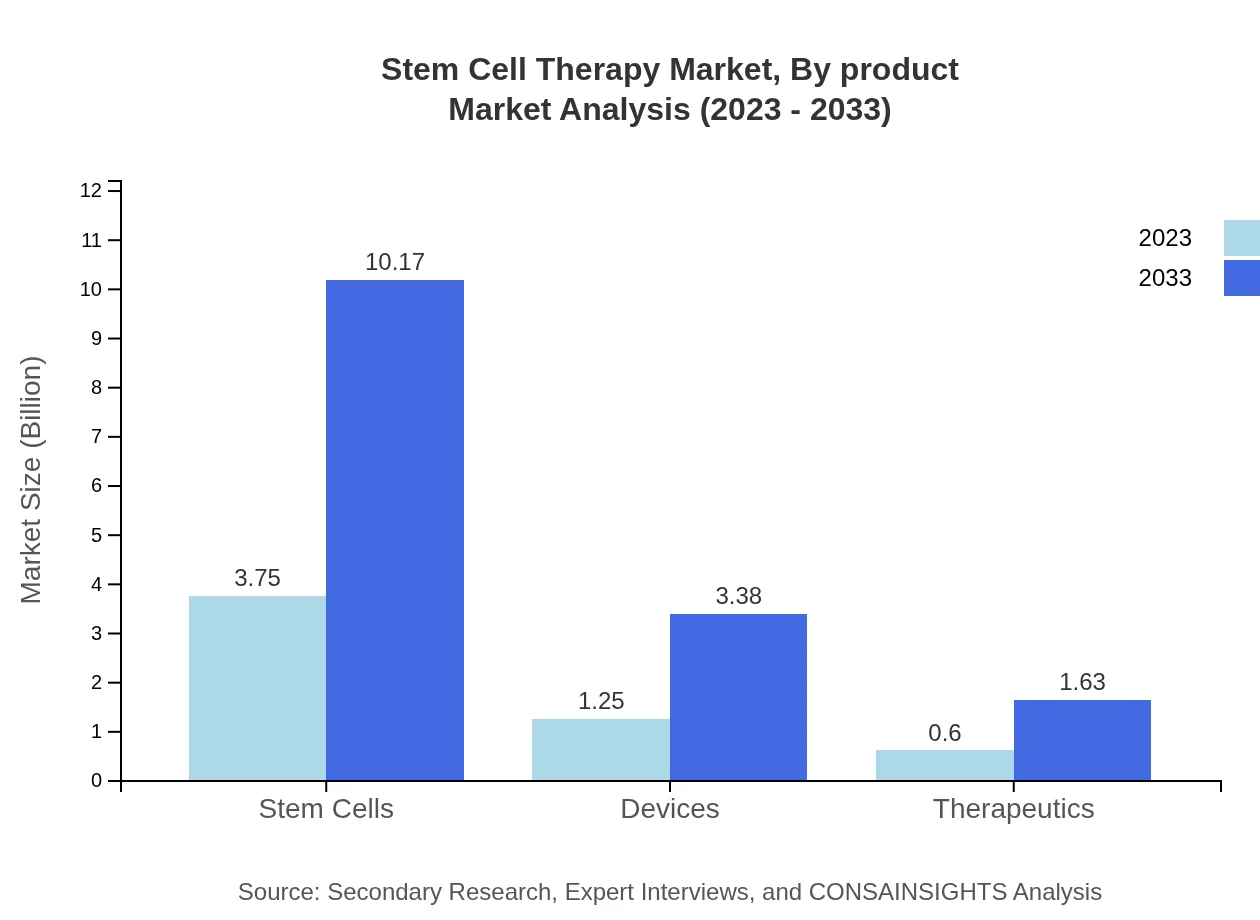
Stem Cell Therapy Market Analysis By Product
The by-product segment indicates that stem cells dominate the market, contributing $3.75 billion in 2023 and expected to grow to $10.17 billion by 2033, while devices and therapeutics segments hold shares of $1.25 billion and $0.60 billion respectively.
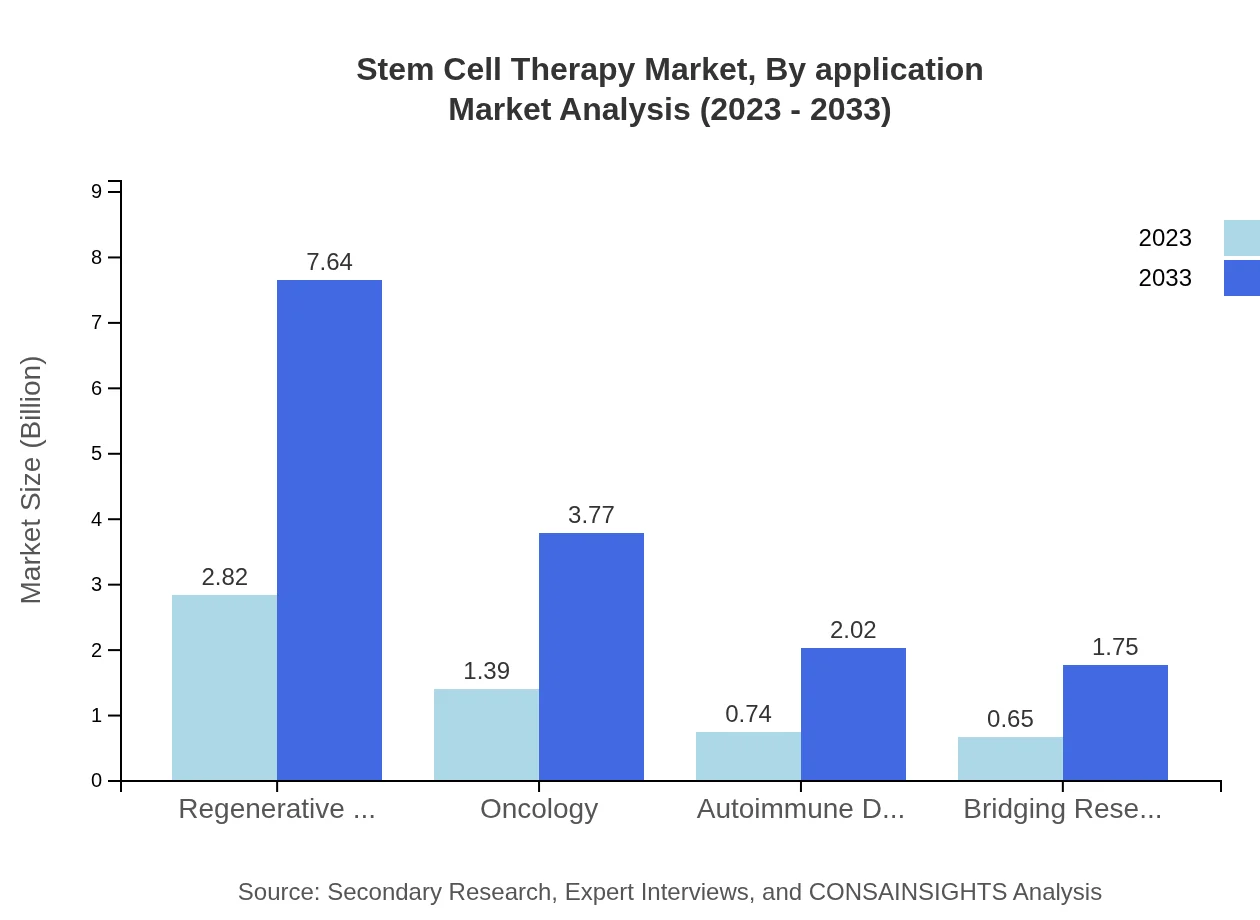
Stem Cell Therapy Market Analysis By Application
In terms of applications, regenerative medicine commands a significant market share, reflecting the increased utilization of stem cells in various therapeutic practices, while oncology and autoimmune disorders also constitute substantial segments of the market.
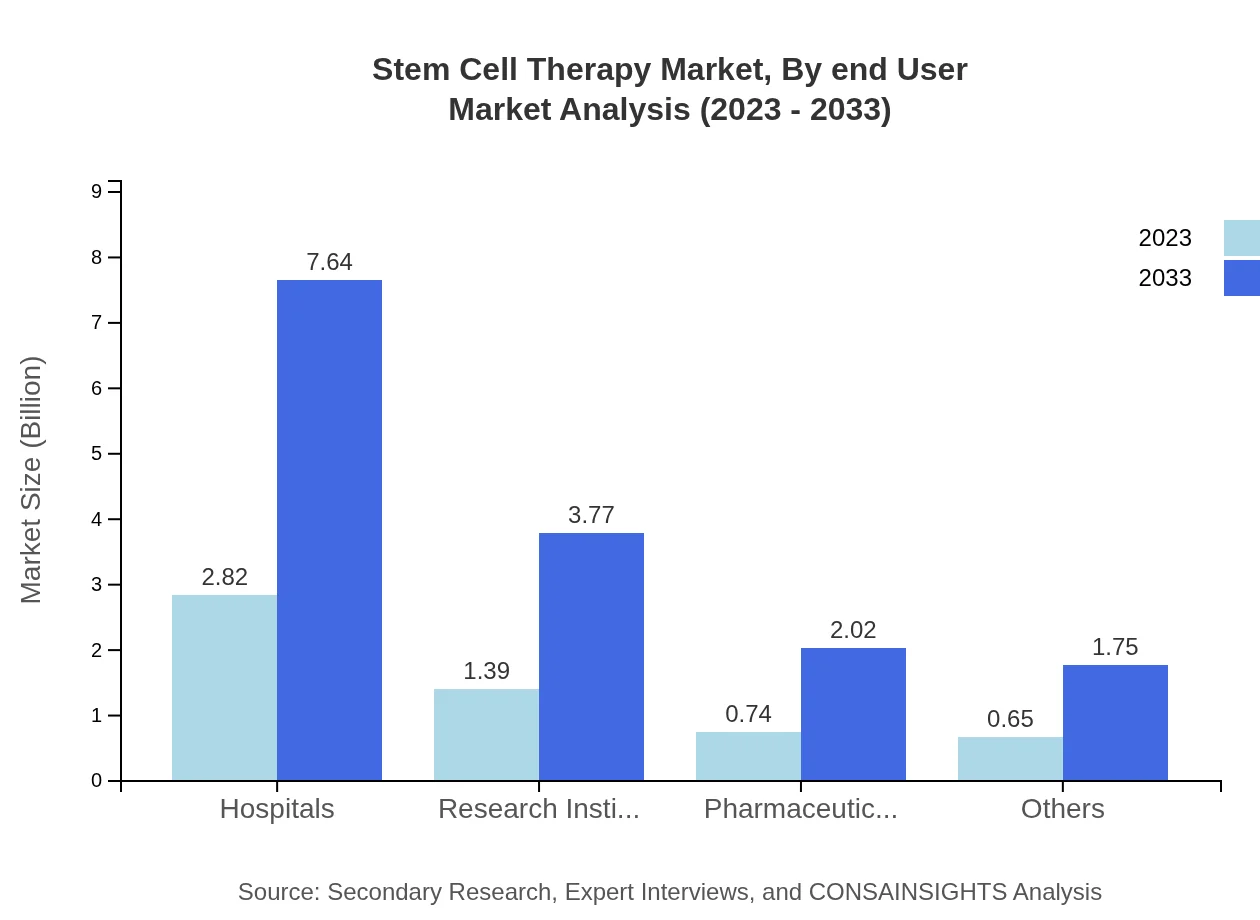
Stem Cell Therapy Market Analysis By End User
Hospitals are the largest end-users of stem cell therapies, with a market size of $2.82 billion in 2023, anticipated to reach $7.64 billion by 2033. Research institutes and pharmaceutical companies also play crucial roles in advancing stem cell applications.
Stem Cell Therapy Market Analysis By Region
Regionally, North America leads the market, followed by Europe and Asia Pacific. This analysis indicates a diverse landscape shaped by varying regulatory environments, technological advancements, and market readiness for stem cell therapies.
Stem Cell Therapy Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Stem Cell Therapy Industry
Mesoblast Limited:
A prominent player specializing in regenerative medicine and innovative cell therapies focusing on chronic pain and cardiovascular diseases.Athersys, Inc.:
Known for its MultiStem® cell therapy product, Athersys focuses on applications in various medical conditions such as stroke and orthopedic diseases.Osiris Therapeutics, Inc.:
A trailblazer in cellular therapy, dedicated to new treatments for conditions that affect various organs through its human cellular product offerings.StemCells, Inc.:
Focusing on the development of stem cell-based therapeutics, especially for neurodegenerative diseases and other conditions.We're grateful to work with incredible clients.









FAQs
What is the market size of stem Cell Therapy?
The global stem cell therapy market size is projected to reach approximately $5.6 billion by 2033, growing from an estimated $2.8 billion in 2023, at a CAGR of 10.1% over this period.
What are the key market players or companies in the stem Cell Therapy industry?
Notable market players in the stem cell therapy industry include major pharmaceutical companies, biotechnology firms, research institutes, and hospitals that are actively engaged in expanding their regenerative medicine portfolios.
What are the primary factors driving the growth in the stem Cell Therapy industry?
The growth of the stem cell therapy industry is driven by advancements in regenerative medicine, increasing prevalence of chronic diseases, heightened research funding, and a growing acceptance of stem cell treatments among healthcare professionals and patients.
Which region is the fastest Growing in the stem Cell Therapy?
North America is the fastest-growing region in the stem cell therapy market, projected to grow from $2.14 billion in 2023 to $5.79 billion by 2033, significantly contributing to the overall global market expansion.
Does ConsaInsights provide customized market report data for the stem Cell Therapy industry?
Yes, ConsaInsights offers customized market report data tailored to the specific needs and requirements of clients within the stem cell therapy industry, ensuring detailed insights into market dynamics.
What deliverables can I expect from this stem Cell Therapy market research project?
Deliverables from the stem cell therapy market research project typically include comprehensive reports, analytical dashboards, detailed market forecasts, competitive landscape analysis, and tailored insights specific to client interests.
What are the market trends of stem Cell Therapy?
Key market trends in stem cell therapy include an increasing focus on personalized medicine, rising investment in research and development, collaborations between biotech firms and research institutes, and a shift towards outpatient treatment alternatives for stem cell therapies.