Sterility Testing Market Report
Published Date: 31 January 2026 | Report Code: sterility-testing
Sterility Testing Market Size, Share, Industry Trends and Forecast to 2033
This report delves into the Sterility Testing market, providing insights into current conditions, market dynamics, and forecasts from 2023 to 2033. It covers market size, growth projections, regional analyses, and a detailed breakdown of products and segments within the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
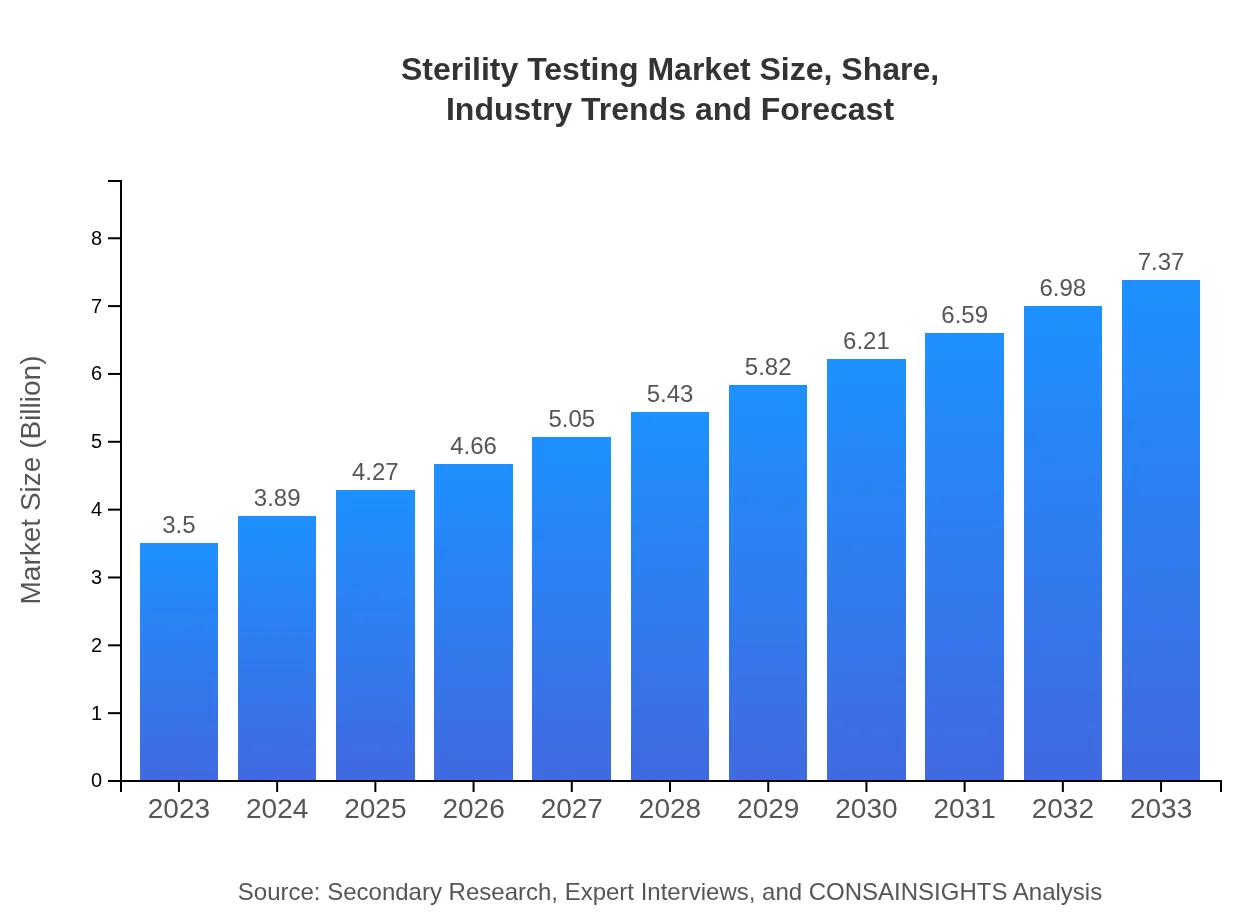
| 2023 Market Size | $3.50 Billion |
| CAGR (2023-2033) | 7.5% |
| 2033 Market Size | $7.37 Billion |
| Top Companies | Thermo Fisher Scientific, Merck KGaA, Charles River Laboratories, Becton, Dickinson and Company |
| Last Modified Date | 31 January 2026 |
Sterility Testing Market Overview
Customize Sterility Testing Market Report market research report
- ✔ Get in-depth analysis of Sterility Testing market size, growth, and forecasts.
- ✔ Understand Sterility Testing's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Sterility Testing
What is the Market Size & CAGR of Sterility Testing market in 2023?
Sterility Testing Industry Analysis
Sterility Testing Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Sterility Testing Market Analysis Report by Region
Europe Sterility Testing Market Report:
The European Sterility Testing market is anticipated to grow from $0.93 billion in 2023 to $1.95 billion by 2033. The region's strong regulatory environment focusing on healthcare and pharmaceuticals fosters demand for high-quality sterility testing. An increase in biopharmaceutical innovations and collaborations will further influence market dynamics positively.Asia Pacific Sterility Testing Market Report:
In 2023, the Sterility Testing market in Asia Pacific is valued at $0.69 billion and is expected to grow to $1.45 billion by 2033. Factors driving this growth include increased healthcare investments and the rising biotechnology sector. Regulatory enhancements and a heightened focus on manufacturing quality standards are expected to accelerate this market's expansion.North America Sterility Testing Market Report:
North America dominates the Sterility Testing market with a projected size rise from $1.20 billion in 2023 to $2.52 billion in 2033. This region benefits from a robust pharmaceutical landscape, stringent regulatory frameworks, and advanced technological integration in testing. The high level of awareness regarding product quality and safety standards also bolsters market growth.South America Sterility Testing Market Report:
The South American Sterility Testing market is projected to grow from $0.31 billion in 2023 to $0.65 billion in 2033. Increasing investments in pharmaceutical production and a growing emphasis on patient safety and product quality are key drivers. However, economic fluctuations can pose challenges to consistent growth.Middle East & Africa Sterility Testing Market Report:
In 2023, the Middle East and Africa Sterility Testing market is valued at $0.38 billion, with expectations of reaching $0.80 billion by 2033. Increasing improvement in healthcare infrastructure and a focus on biopharmaceutical manufacturing are motivating factors. However, regional challenges like political instability and economic limitations can impact growth prospects.Tell us your focus area and get a customized research report.
Sterility Testing Market Analysis By Product
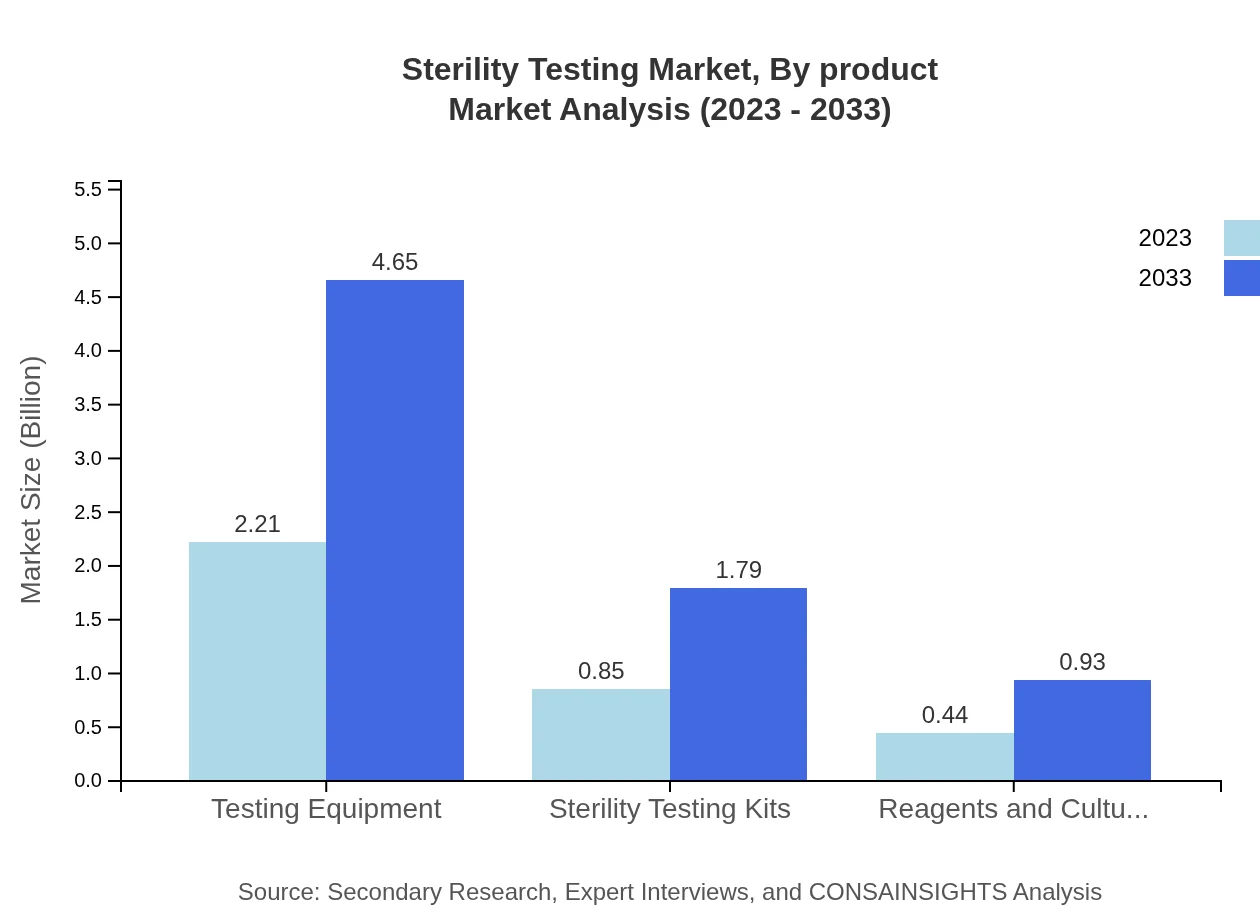
The product segment is dominated by Testing Equipment, which in 2023 holds a market value of $2.21 billion, rising to $4.65 billion by 2033. Membrane Filtration follows, with values from $1.89 billion in 2023 to $3.99 billion by 2033, reflecting its significance in sterility testing. Sterility Testing Kits account for $0.85 billion in 2023 and are expected to rise to $1.79 billion, while Reagents and Culture Media show growth from $0.44 billion to $0.93 billion over the forecast period.
Sterility Testing Market Analysis By Application
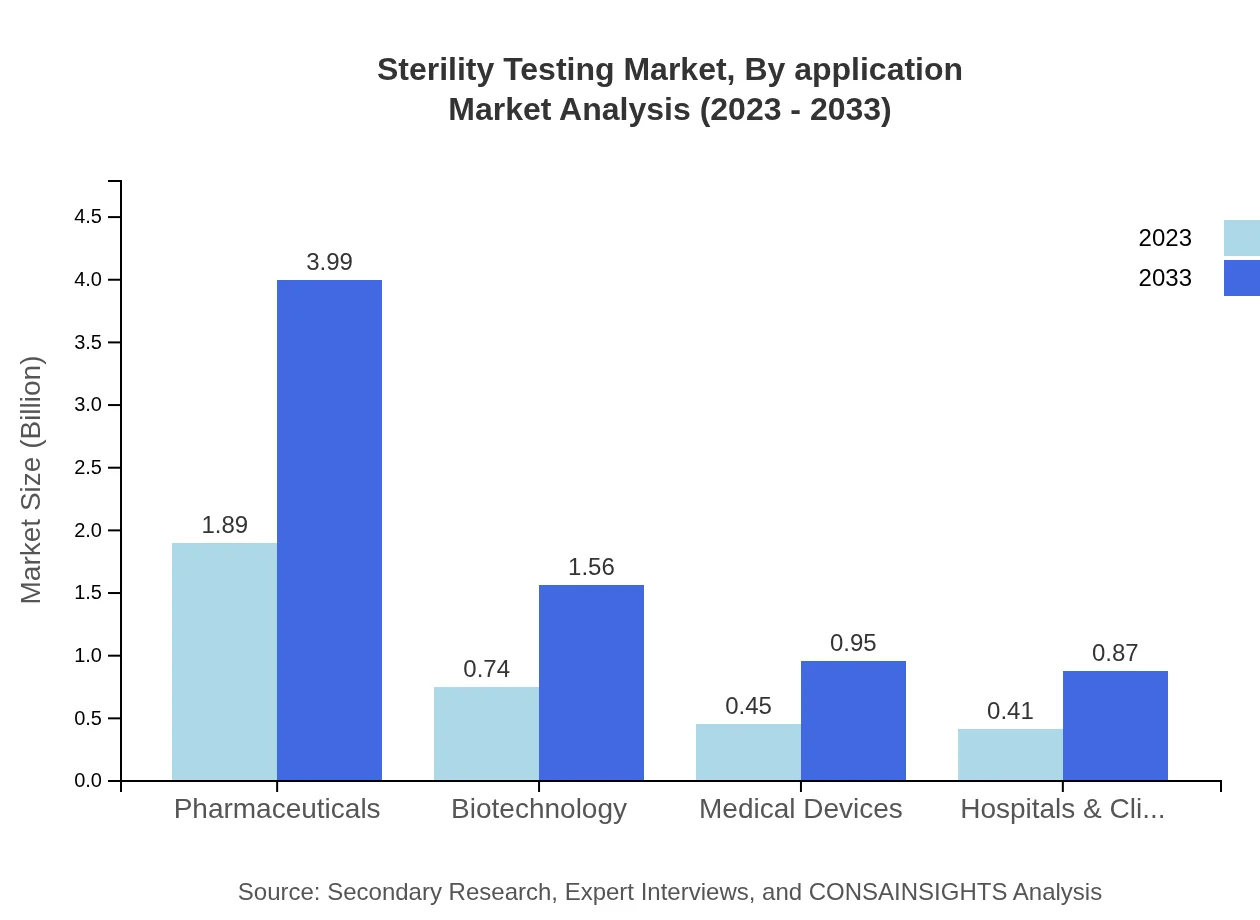
The pharmaceutical application segment commands the largest market share, valued at $1.89 billion in 2023, projected to reach $3.99 billion by 2033. Biotechnology follows, with a size growing from $0.74 billion to $1.56 billion. Hospitals & Clinical Laboratories, and Research Institutions also contribute significantly, with respective forecasts indicating growth from $0.41 billion to $0.87 billion and $0.45 billion to $0.95 billion.
Sterility Testing Market Analysis By Method
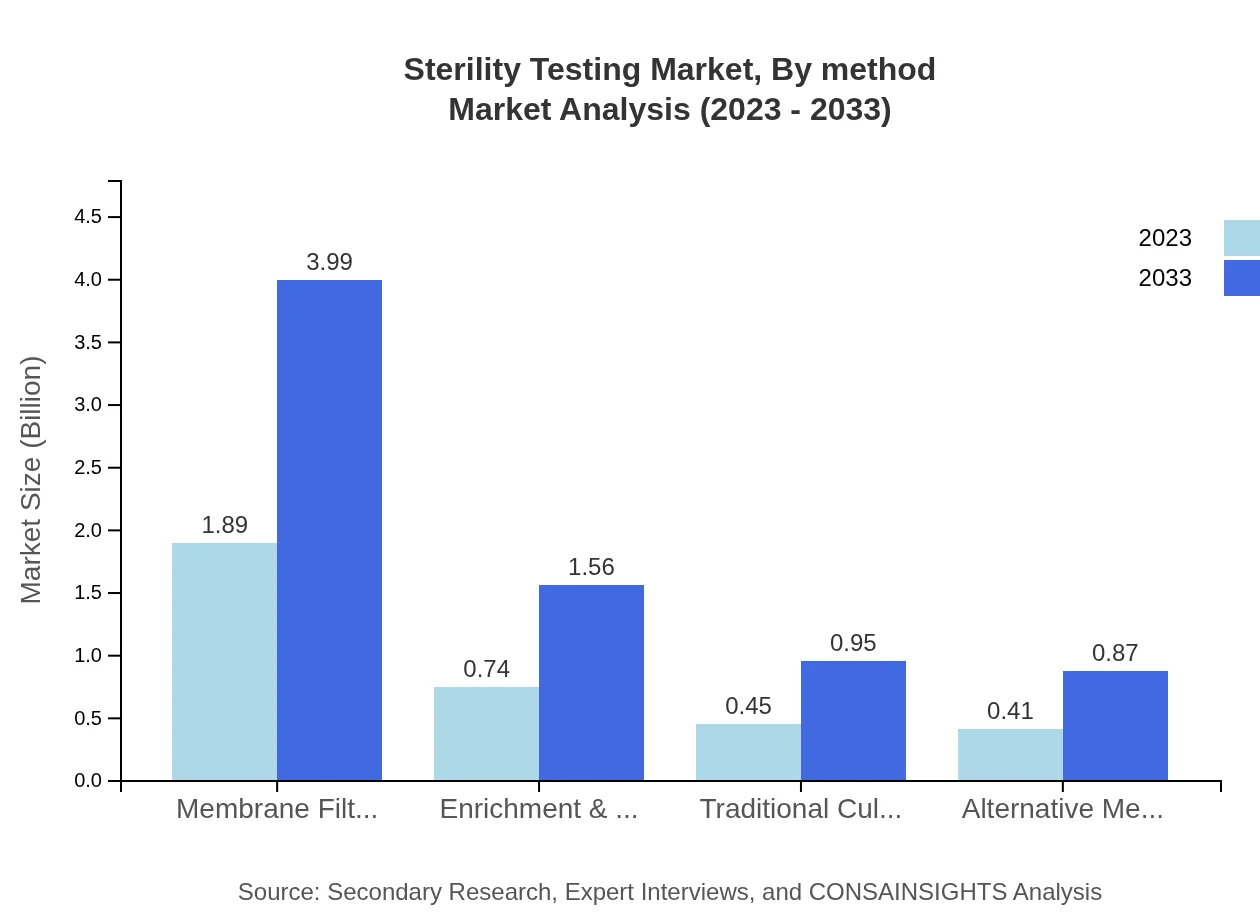
The market analysis by method shows Traditional Culture Methods beginning at $0.45 billion in 2023 and anticipated growth to $0.95 billion by 2033. Alternative Methods are also growing, expanding from $0.41 billion to $0.87 billion, showcasing a trend towards faster testing solutions. This shift reflects the industry's response to market demands for efficiency and speed.
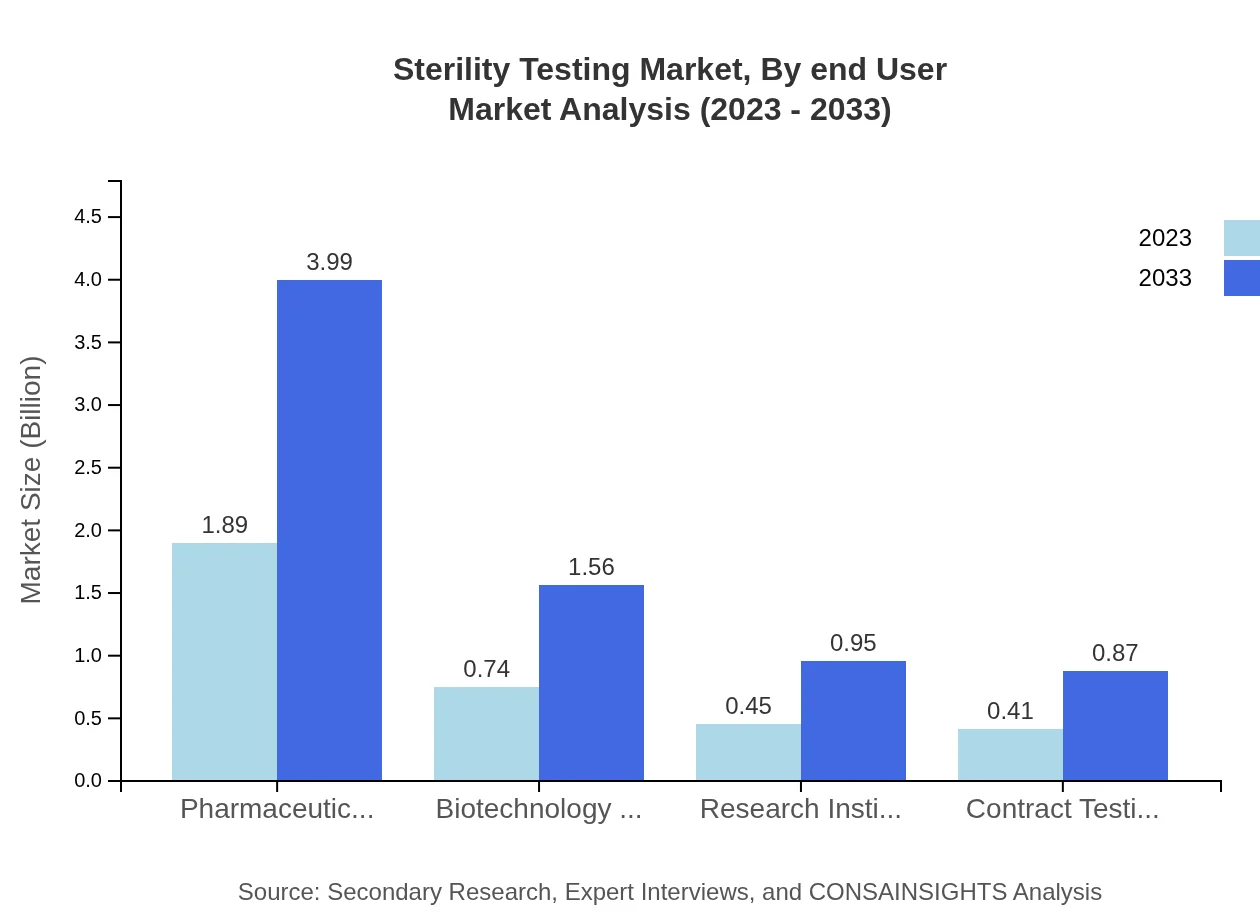
Sterility Testing Market Analysis By End User
The end-user segment is robust, with Pharmaceuticals leading at $1.89 billion in 2023 and projected growth to $3.99 billion. Biotechnology Companies and Contract Testing Laboratories display significant growth, moving from $0.74 billion and $0.41 billion to $1.56 billion and $0.87 billion respectively by 2033. This trend indicates the increasing reliance on third-party testing services across industries.
Sterility Testing Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Sterility Testing Industry
Thermo Fisher Scientific:
A leading provider of analytical instruments, reagents, and consumables. They offer an extensive range of products suited for sterility testing across various applications.Merck KGaA:
Specializes in providing laboratory supplies and reagents. Their testing kits and technologies play a crucial role in sterility assurance processes.Charles River Laboratories:
Provides comprehensive testing services, including sterility testing, to biopharma customers ensuring compliance with global regulatory standards.Becton, Dickinson and Company:
Known for its advanced medical devices, BD also plays a significant role in sterility testing through its innovative solutions and services.We're grateful to work with incredible clients.









FAQs
What is the market size of sterility Testing?
The sterility testing market is projected to grow from $3.5 billion in 2023 to approximately $7.38 billion by 2033, with a CAGR of 7.5% over the forecast period.
What are the key market players or companies in this sterility Testing industry?
Key players in the sterility testing market include major companies like Thermo Fisher Scientific, Merck KGaA, and Sartorius AG, which provide innovative technologies and solutions for sterility testing in pharmaceuticals and biotechnology.
What are the primary factors driving the growth in the sterility Testing industry?
Growth in the sterility testing industry is driven by increasing pharmaceutical production, stringent regulatory standards for product safety, and rising investments in biotechnology research, necessitating reliable sterility testing methods.
Which region is the fastest Growing in the sterility Testing?
North America is the fastest-growing region in the sterility testing market, projected to increase from $1.20 billion in 2023 to $2.52 billion by 2033, fueled by advancements in biopharmaceuticals.
Does ConsaInsights provide customized market report data for the sterility Testing industry?
Yes, ConsaInsights offers customized market reports tailored to specific needs in the sterility testing industry, providing in-depth insights and flexible data solutions for businesses.
What deliverables can I expect from this sterility Testing market research project?
Deliverables from the sterility testing market research project include comprehensive reports, detailed data analysis, market forecasts, and actionable insights, including segment-wise breakdowns.
What are the market trends of sterility Testing?
Current trends in the sterility testing market include a shift towards rapid testing methods, increased adoption of automated systems, and a stronger focus on regulatory compliance and quality assurance.