Structural Heart Devices Market Report
Published Date: 31 January 2026 | Report Code: structural-heart-devices
Structural Heart Devices Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Structural Heart Devices market, including insights into market trends, size, segmentation, and forecasts from 2023 to 2033. It aims to equip stakeholders with data-driven insights to make informed decisions.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
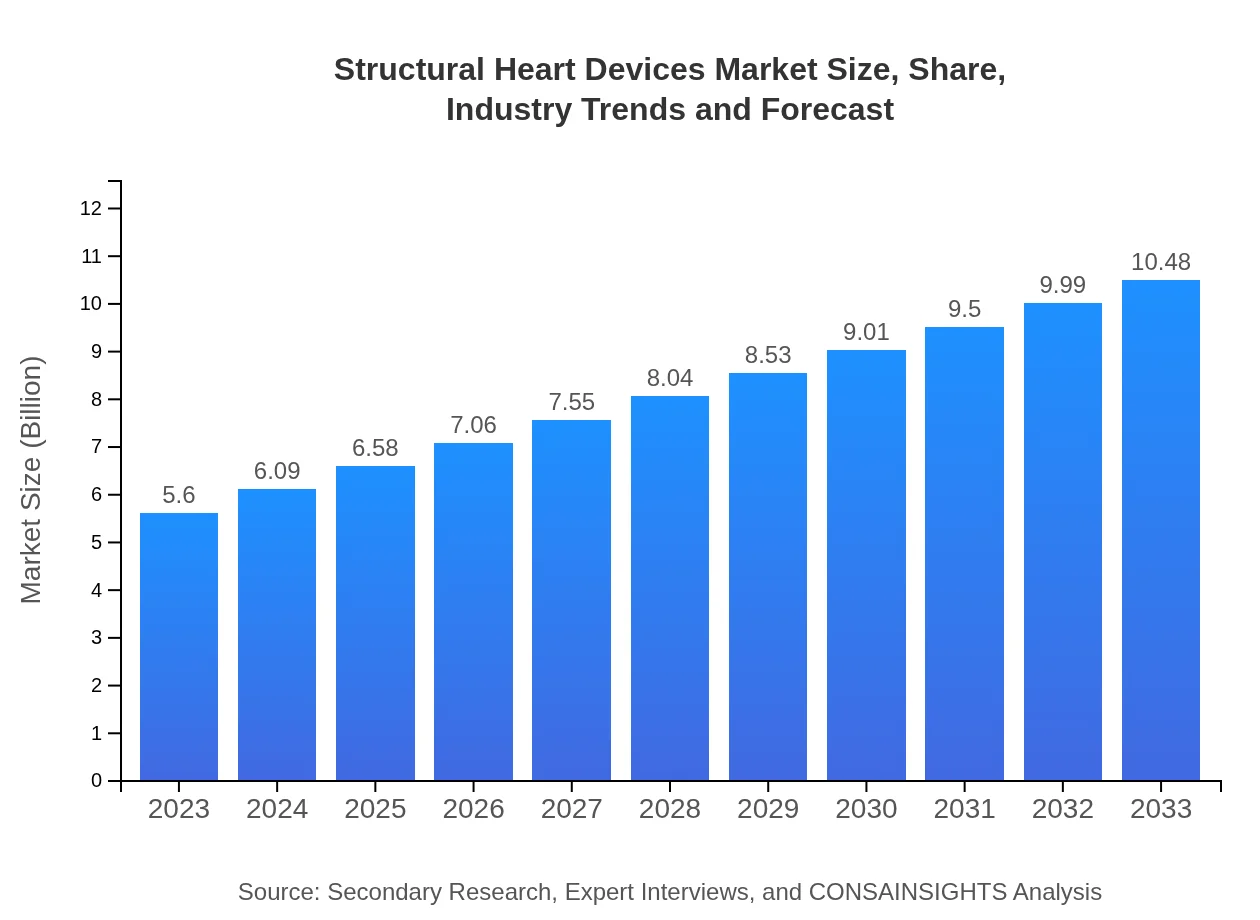
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 6.3% |
| 2033 Market Size | $10.48 Billion |
| Top Companies | Medtronic , Edwards Lifesciences, Abbott Laboratories, Boston Scientific, Johnson & Johnson |
| Last Modified Date | 31 January 2026 |
Structural Heart Devices Market Overview
Customize Structural Heart Devices Market Report market research report
- ✔ Get in-depth analysis of Structural Heart Devices market size, growth, and forecasts.
- ✔ Understand Structural Heart Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Structural Heart Devices
What is the Market Size & CAGR of Structural Heart Devices market in 2023?
Structural Heart Devices Industry Analysis
Structural Heart Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Structural Heart Devices Market Analysis Report by Region
Europe Structural Heart Devices Market Report:
Europe's market size is expected to grow from $1.67 billion in 2023 to $3.13 billion by 2033. The region is experiencing a rising demand for advanced cardiac therapies, with regulatory support promoting the swift adoption of innovative devices.Asia Pacific Structural Heart Devices Market Report:
The Asia Pacific region is witnessing significant growth in the Structural Heart Devices market, driven by an increasing aging population and a rise in cardiovascular diseases. The market size is projected to grow from $1.06 billion in 2023 to $1.99 billion by 2033, reflecting a robust adoption of minimally invasive techniques.North America Structural Heart Devices Market Report:
North America, holding the largest market share, is expected to grow from $2.02 billion in 2023 to $3.79 billion by 2033. Factors such as advanced healthcare facilities and a high prevalence of heart diseases drive this market, with hospitals being the primary end-users.South America Structural Heart Devices Market Report:
In South America, the market is relatively smaller, projected to grow from $0.52 billion in 2023 to $0.98 billion by 2033. While healthcare infrastructure improvements are expected to contribute to growth, access and affordability remain challenges.Middle East & Africa Structural Heart Devices Market Report:
The market in the Middle East and Africa is projected to grow from $0.32 billion in 2023 to $0.59 billion by 2033, benefitting from improvements in healthcare facilities and initiatives aimed at enhancing cardiac care in underserved areas.Tell us your focus area and get a customized research report.
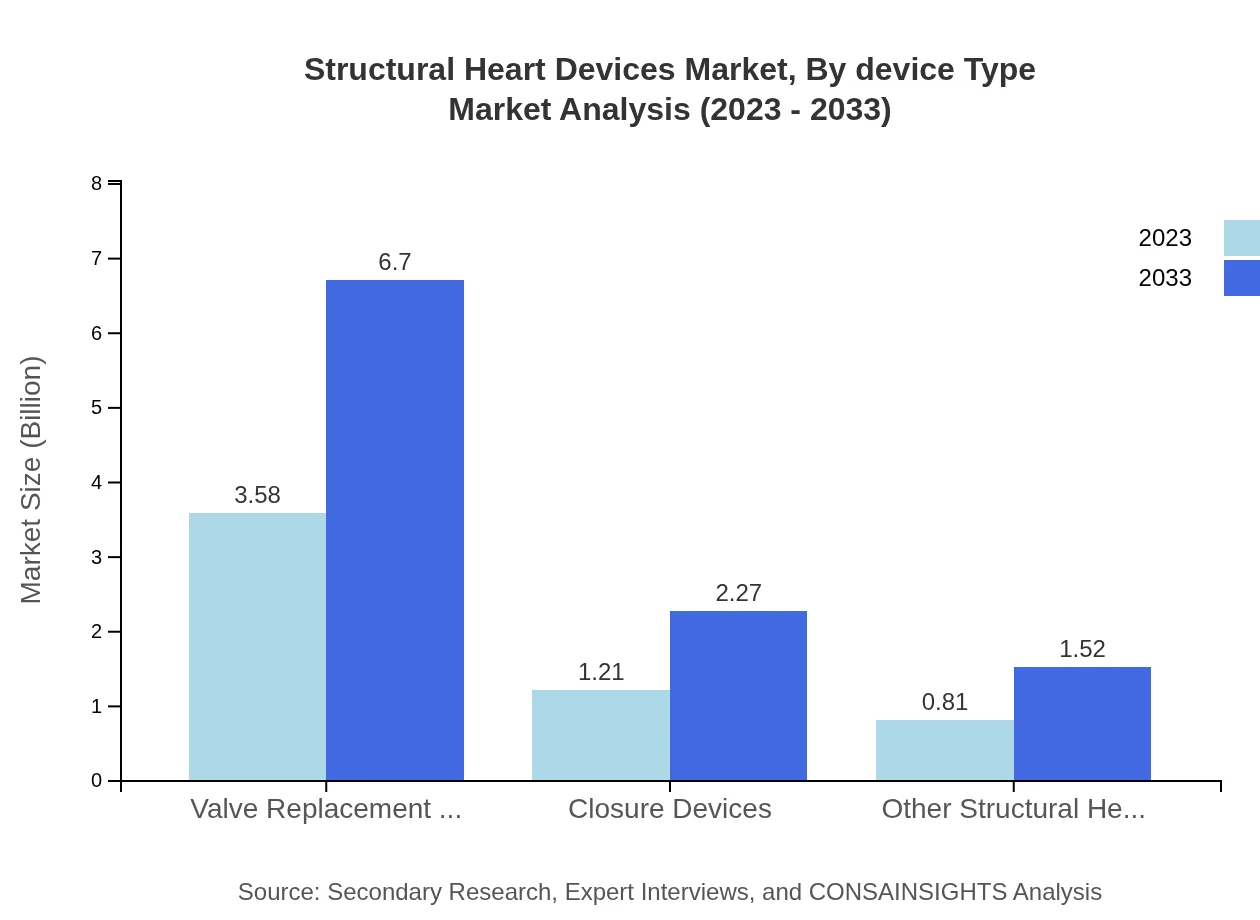
Structural Heart Devices Market Analysis By Device Type
The Structural Heart Devices market is dominated by valve replacement devices, projected to grow from $3.58 billion in 2023 to $6.70 billion by 2033. Closure devices follow with a size of $1.21 billion, expected to reach $2.27 billion, while other devices will expand from $0.81 billion to $1.52 billion during the forecast period.
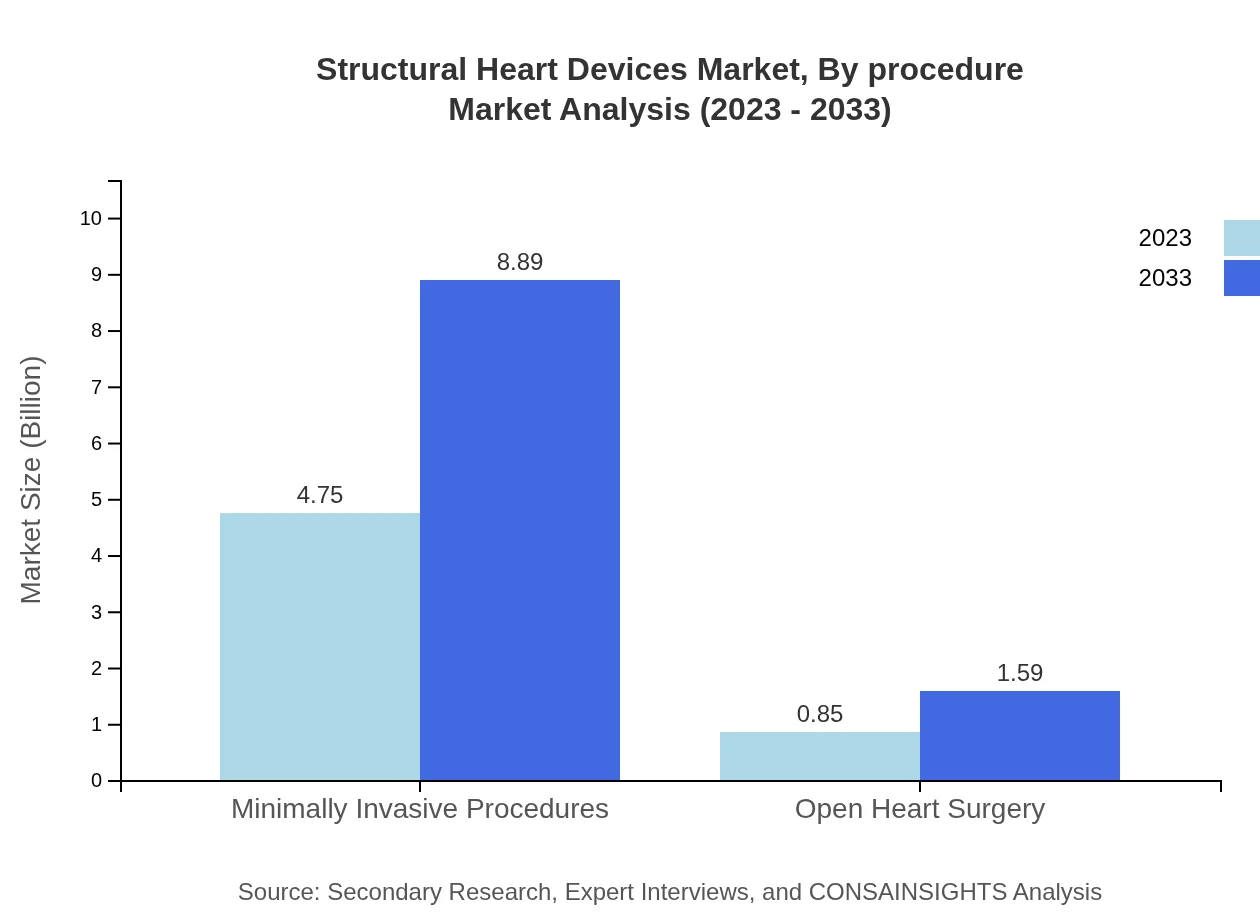
Structural Heart Devices Market Analysis By Procedure
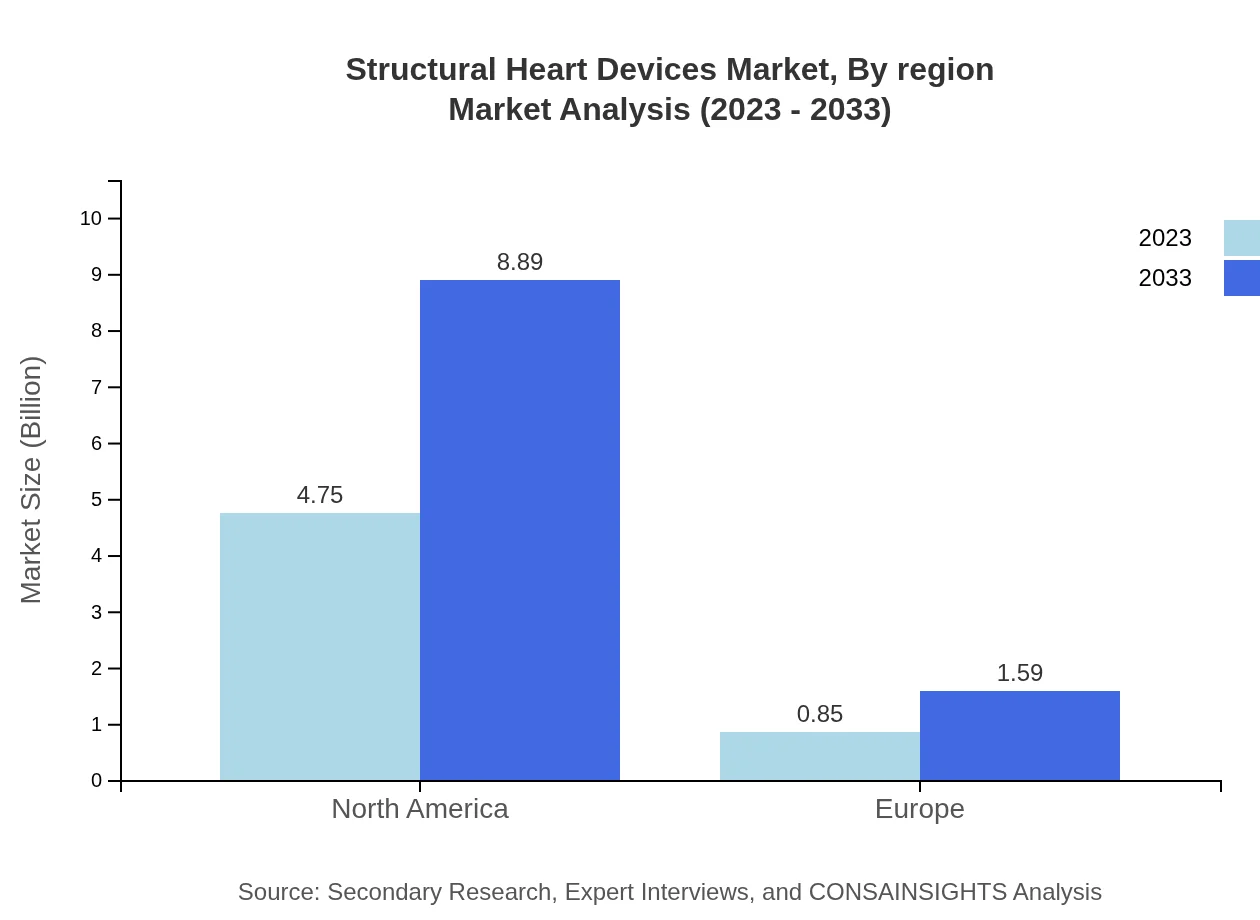
Minimally invasive procedures represent a significant share, with market valuations of $4.75 billion in 2023 and $8.89 billion in 2033. Open heart surgery, while traditional, is projected to grow modestly from $0.85 billion to $1.59 billion over the same period.
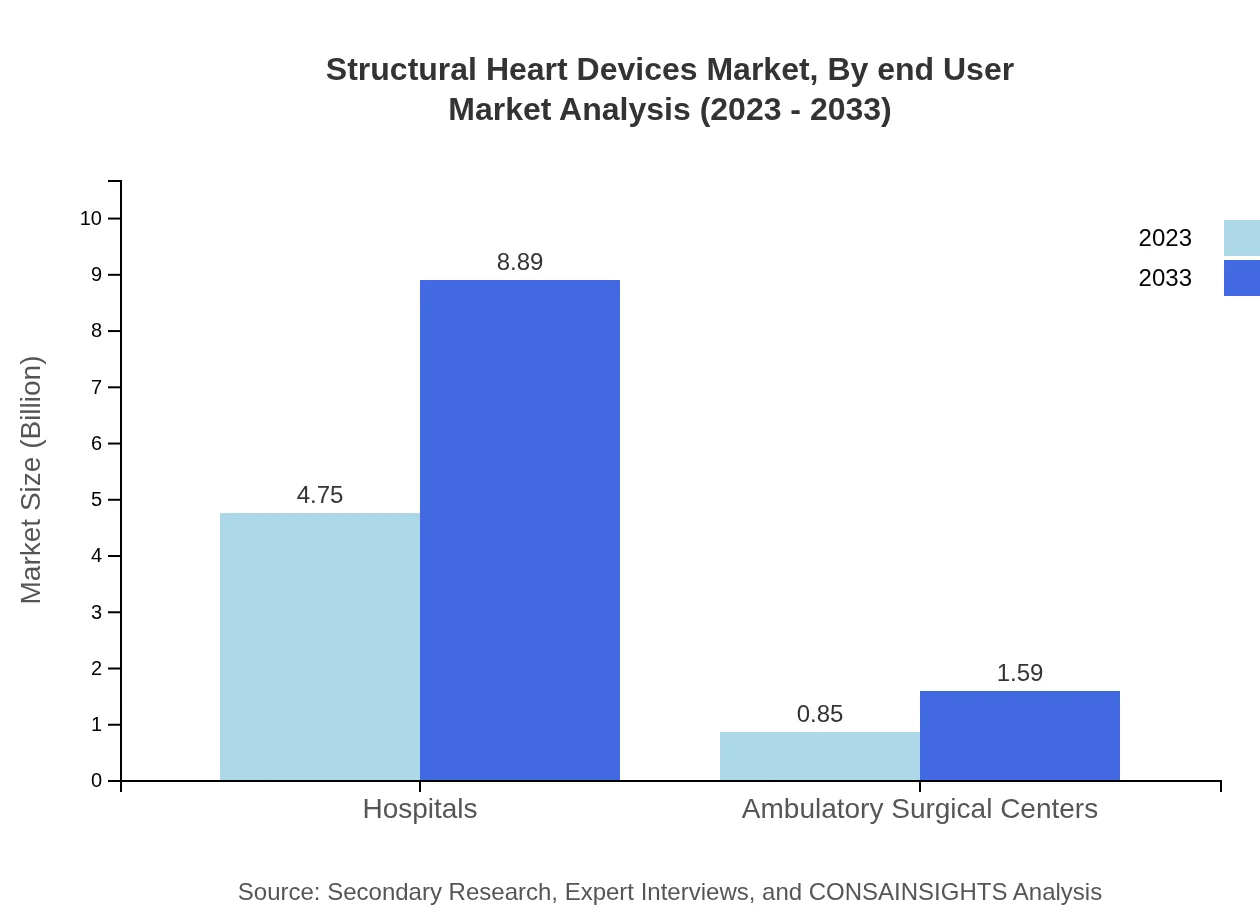
Structural Heart Devices Market Analysis By End User
Hospitals dominate the Structural Heart Devices market, expected to maintain a size from $4.75 billion in 2023 to $8.89 billion by 2033. Ambulatory surgical centers are also growing, with projections of $0.85 billion to $1.59 billion.
Structural Heart Devices Market Analysis By Region
Regional analysis shows that North America leads the market by a significant margin, followed by Europe and Asia Pacific. Emerging markets in South America and the Middle East and Africa show potential for growth, albeit from a smaller base.
Structural Heart Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Structural Heart Devices Industry
Medtronic :
A global leader in medical technology, Medtronic is renowned for its innovative valve replacement devices, helping to advance cardiac care worldwide.Edwards Lifesciences:
Specializing in heart valve technologies, Edwards Lifesciences offers a range of solutions that enhance patient outcomes in structural heart treatments.Abbott Laboratories:
Abbott's diverse portfolio includes cutting-edge devices for structural heart interventions, contributing to the growing acceptance of minimally invasive procedures.Boston Scientific:
Known for its versatility and innovation, Boston Scientific provides a comprehensive range of structural heart devices, targeting various cardiac conditions effectively.Johnson & Johnson:
With a strong presence in the cardiovascular market, Johnson & Johnson is committed to developing innovative solutions in the structural heart devices sector.We're grateful to work with incredible clients.









FAQs
What is the market size of structural Heart Devices?
The global structural heart devices market is projected to reach approximately $5.6 billion in 2023, with an anticipated CAGR of 6.3%, expanding towards $8.9 billion by 2033, driven by growing cardiovascular diseases and advancements in device technology.
What are the key market players or companies in the structural Heart Devices industry?
Key players in the structural heart devices market include leading companies such as Medtronic, Abbott Laboratories, Edwards Lifesciences, Boston Scientific, and JenaValve Technology. These companies are at the forefront of innovation, providing a range of advanced products in this sector.
What are the primary factors driving the growth in the structural Heart Devices industry?
Growth in the structural heart devices industry is primarily driven by the increasing prevalence of heart diseases, technological advancements in minimally invasive procedures, and the aging population. Additionally, rising healthcare expenditure and improved reimbursement policies contribute to market expansion.
Which region is the fastest Growing in the structural Heart Devices?
The fastest-growing region in the structural heart devices market is North America, projected to grow from $2.02 billion in 2023 to $3.79 billion by 2033. Europe and Asia Pacific also show significant growth, reflecting increased healthcare access and technological adoption.
Does ConsaInsights provide customized market report data for the structural Heart Devices industry?
Yes, Consainsights offers customized market report data for the structural heart devices industry. We can tailor reports to meet specific client needs, incorporating unique insights, data analytics, and projections for targeted market segments.
What deliverables can I expect from this structural Heart Devices market research project?
Deliverables from the structural heart devices market research project include comprehensive reports with market size, growth forecasts, competitive analysis, segmentation insights, and regional trends, ensuring clients receive clear and actionable information.
What are the market trends of structural Heart Devices?
Current market trends in the structural heart devices sector include a shift towards minimally invasive surgical options, increasing adoption of innovative technologies such as bioresorbable stents, and ongoing research into new device types and improved patient outcomes.