Surgical Kits Market Report
Published Date: 31 January 2026 | Report Code: surgical-kits
Surgical Kits Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive overview of the Surgical Kits market, with insights into market size, CAGR, segmentation, regional analysis, technology advancements, and major global players. The forecast period analyzed is from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
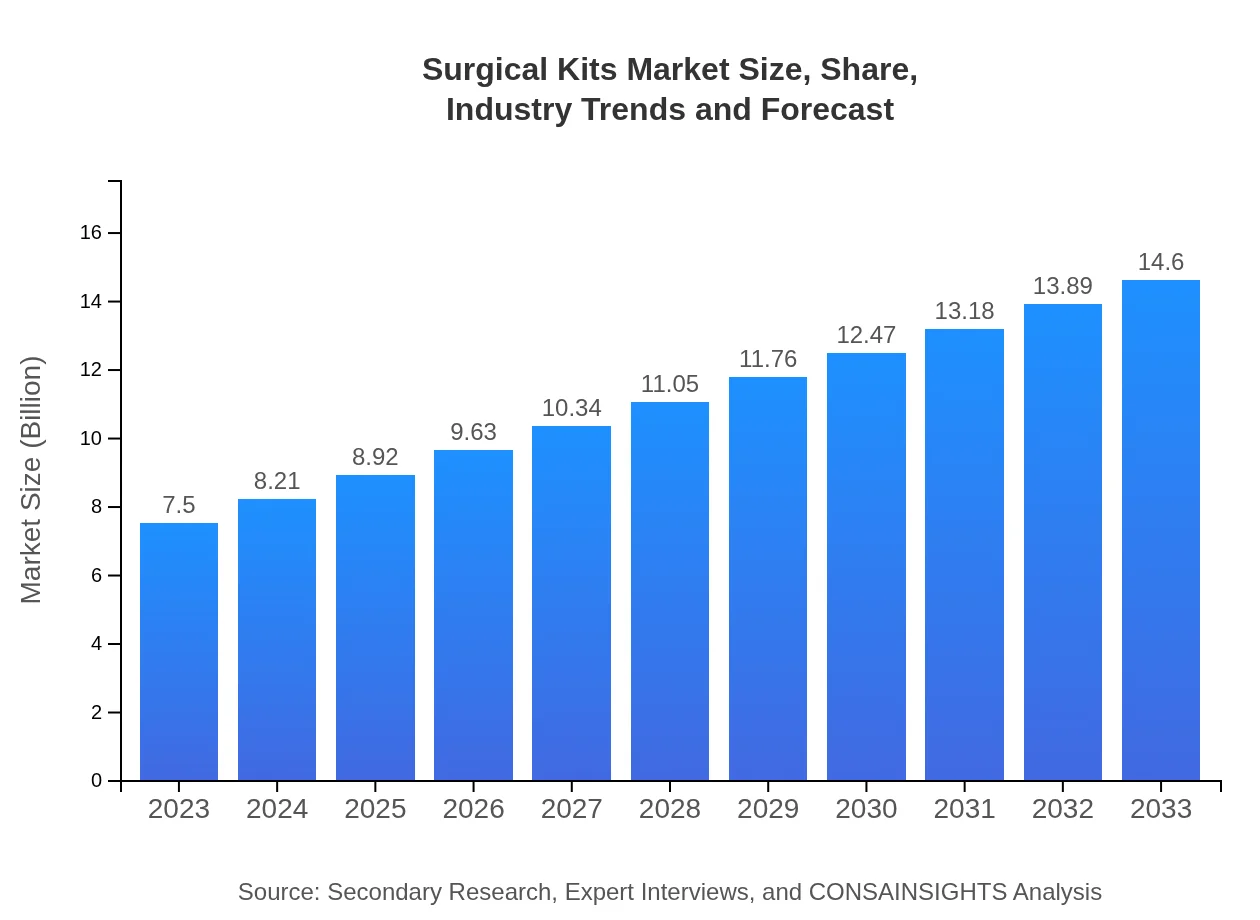
| 2023 Market Size | $7.50 Billion |
| CAGR (2023-2033) | 6.7% |
| 2033 Market Size | $14.60 Billion |
| Top Companies | Medtronic , Johnson & Johnson, 3M Health Care, B. Braun, Cardinal Health |
| Last Modified Date | 31 January 2026 |
Surgical Kits Market Overview
Customize Surgical Kits Market Report market research report
- ✔ Get in-depth analysis of Surgical Kits market size, growth, and forecasts.
- ✔ Understand Surgical Kits's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Surgical Kits
What is the Market Size & CAGR of Surgical Kits market in 2023?
Surgical Kits Industry Analysis
Surgical Kits Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Surgical Kits Market Analysis Report by Region
Europe Surgical Kits Market Report:
The European market is expected to grow from $2.62 billion in 2023 to $5.09 billion by 2033. Factors include a well-established healthcare system and a rising geriatric population which necessitates more surgical procedures.Asia Pacific Surgical Kits Market Report:
In the Asia Pacific region, the surgical kits market is projected to rise from $1.37 billion in 2023 to $2.67 billion by 2033. The growth is attributed to increasing healthcare infrastructure investments and rising surgical procedures in countries like China and India. Moreover, the growing population and associated healthcare needs are driving demand.North America Surgical Kits Market Report:
North America dominates the market, with a valuation of $2.44 billion in 2023, projected to reach $4.75 billion by 2033. Factors contributing to this growth include advanced healthcare infrastructure, high surgery prevalence, and a preference for innovative surgical solutions.South America Surgical Kits Market Report:
Latin America's market is expected to grow from $0.22 billion in 2023 to $0.42 billion by 2033. The increase is driven primarily by expanding healthcare access and government initiatives aiming to improve surgical capabilities, particularly in Brazil and Argentina.Middle East & Africa Surgical Kits Market Report:
In the Middle East and Africa, the surgical kits market is projected to grow from $0.85 billion in 2023 to $1.66 billion by 2033. The expansion is driven by increasing healthcare investments and the need for improved surgical practices.Tell us your focus area and get a customized research report.
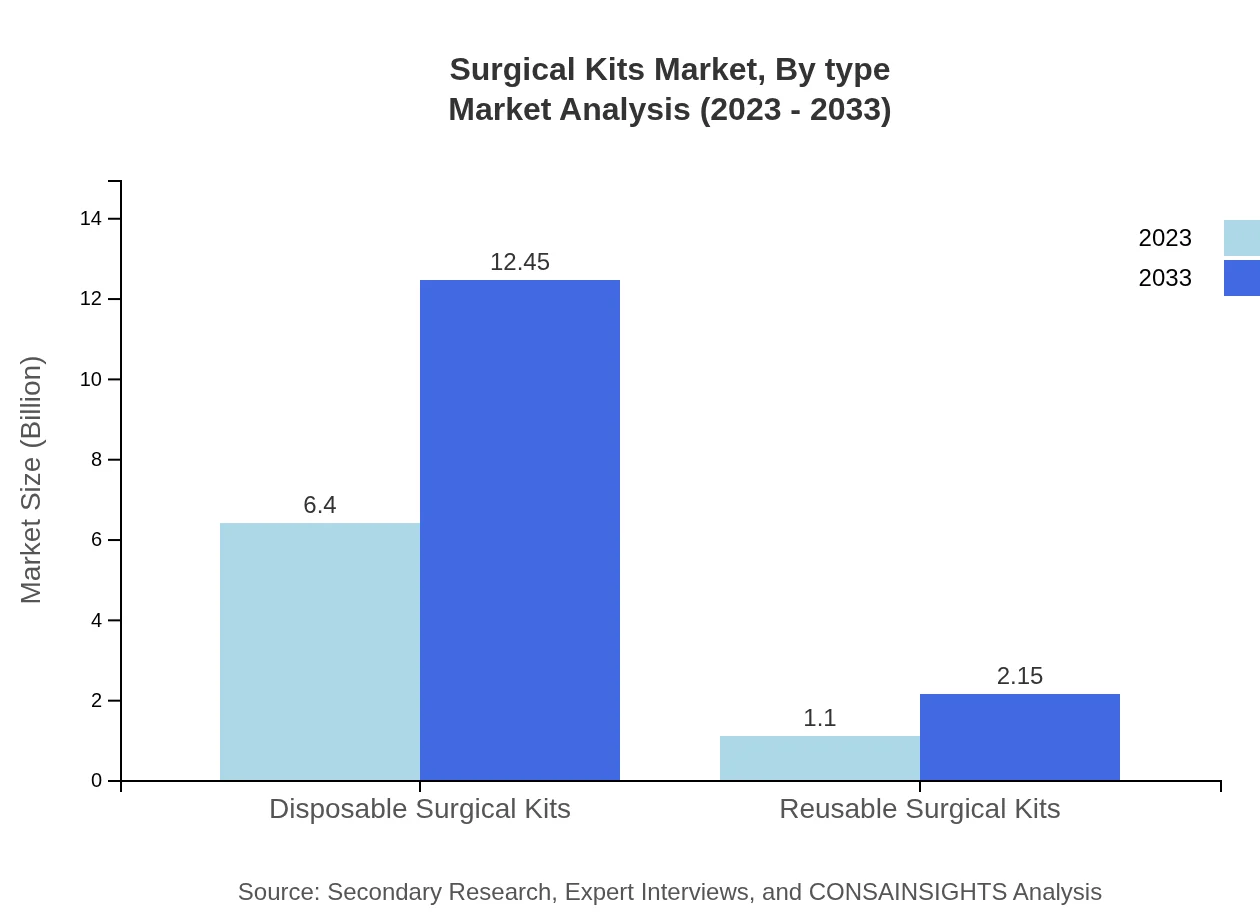
Surgical Kits Market Analysis By Type
The global surgical kits market is primarily segmented into disposable and reusable kits. Disposable surgical kits, projected to have a market size of $6.40 billion in 2023 and $12.45 billion by 2033, dominate the market due to their convenience and lower risk of cross-contamination. On the other hand, reusable surgical kits accounted for a market size of $1.10 billion in 2023 and are expected to reach $2.15 billion by 2033. Although they have a smaller market share, their sustainability factor appeals to budget-conscious end-users.
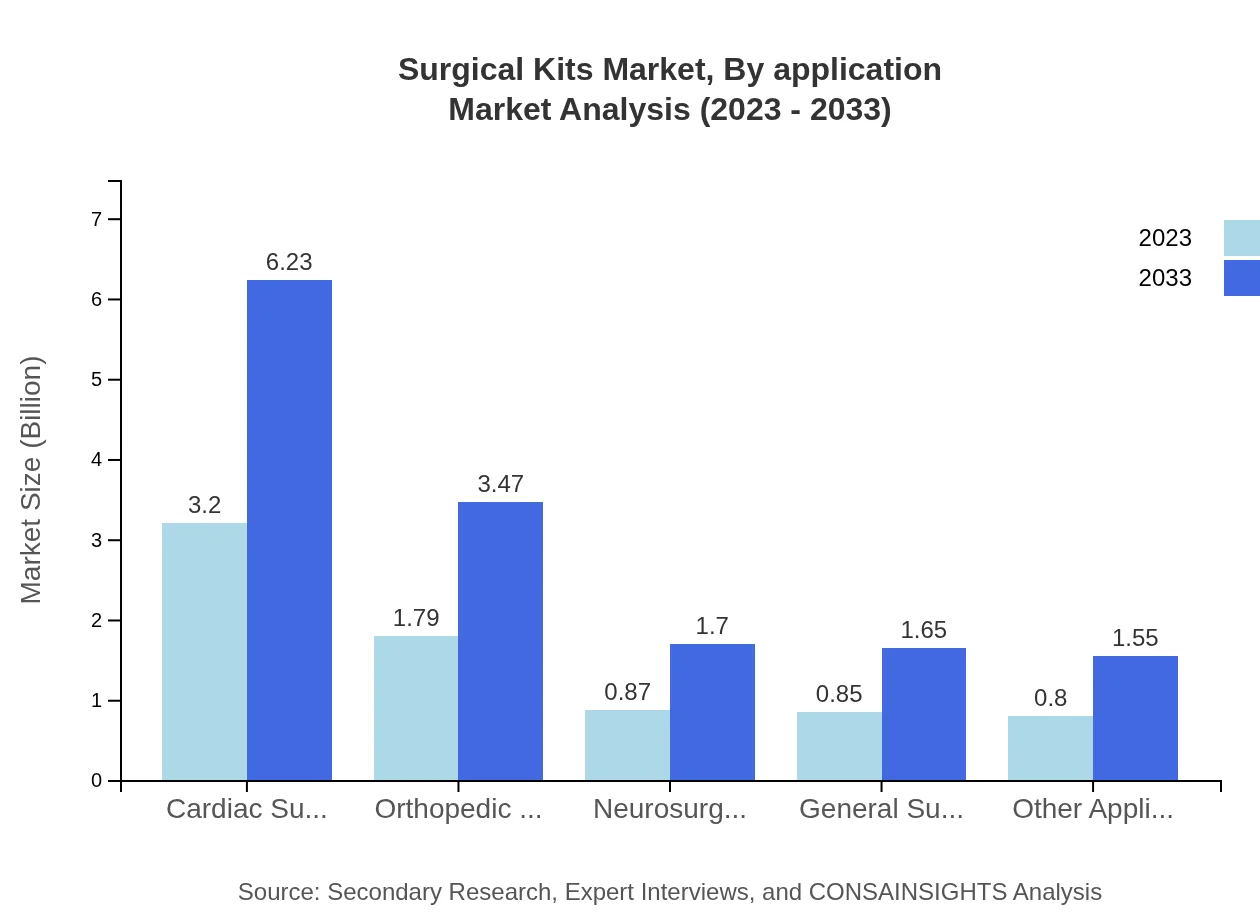
Surgical Kits Market Analysis By Application
The applications can be categorized into cardiac surgery ($3.20 billion in 2023), orthopedic surgery ($1.79 billion), neurosurgery ($0.87 billion), general surgery ($0.85 billion), and other applications ($0.80 billion). The cardiac surgery segment holds 42.67% market share as of 2023, and is expected to expand significantly due to an increase in cardiac diseases and minimally invasive surgical procedures.
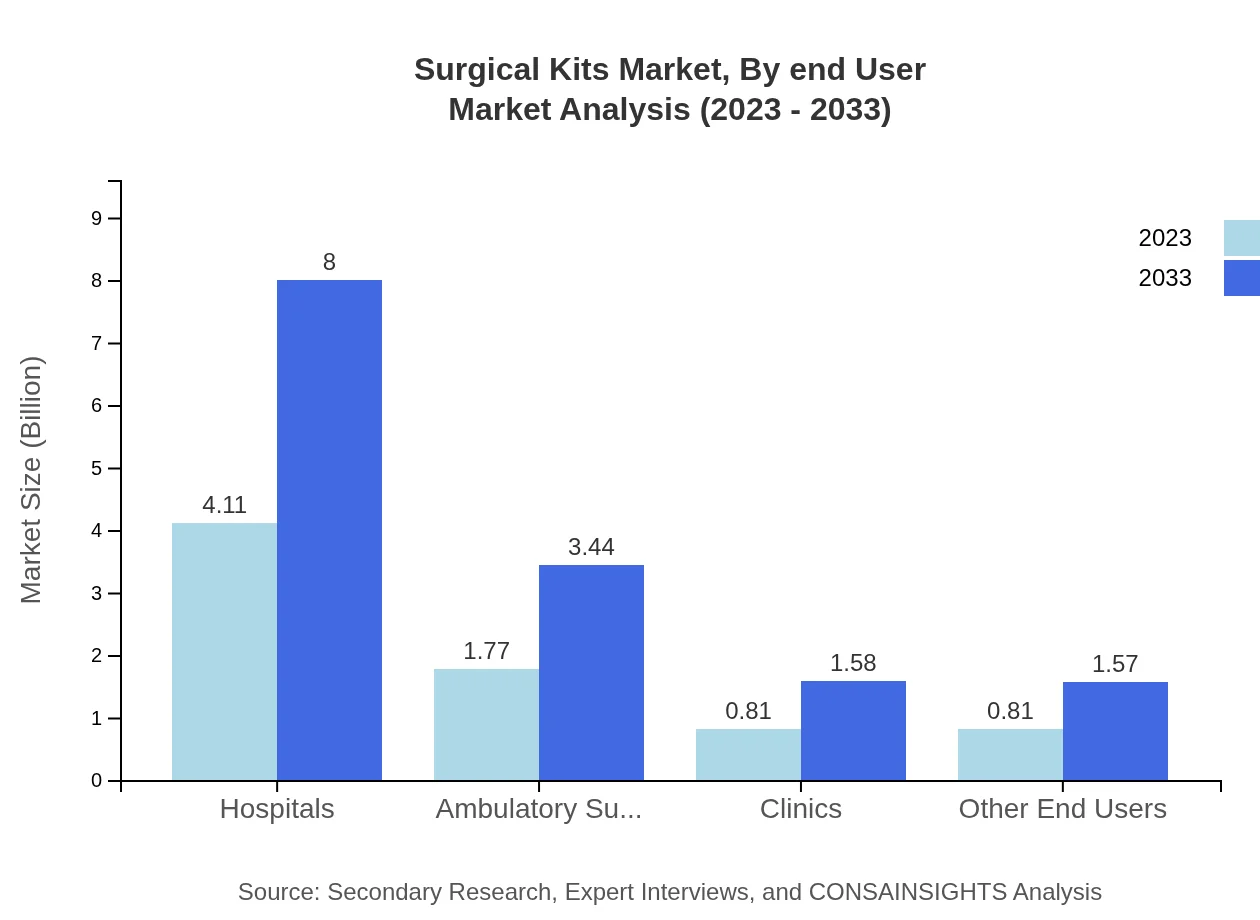
Surgical Kits Market Analysis By End User
The end-user segmentation of the surgical kits market includes hospitals, ambulatory surgical centers, clinics, and other entities. Hospitals lead the market with a size of $4.11 billion in 2023, followed by ambulatory surgical centers at $1.77 billion and clinics at $0.81 billion. The hospitals segment's significant market share can be attributed to the high volume of surgical procedures being performed in these facilities.
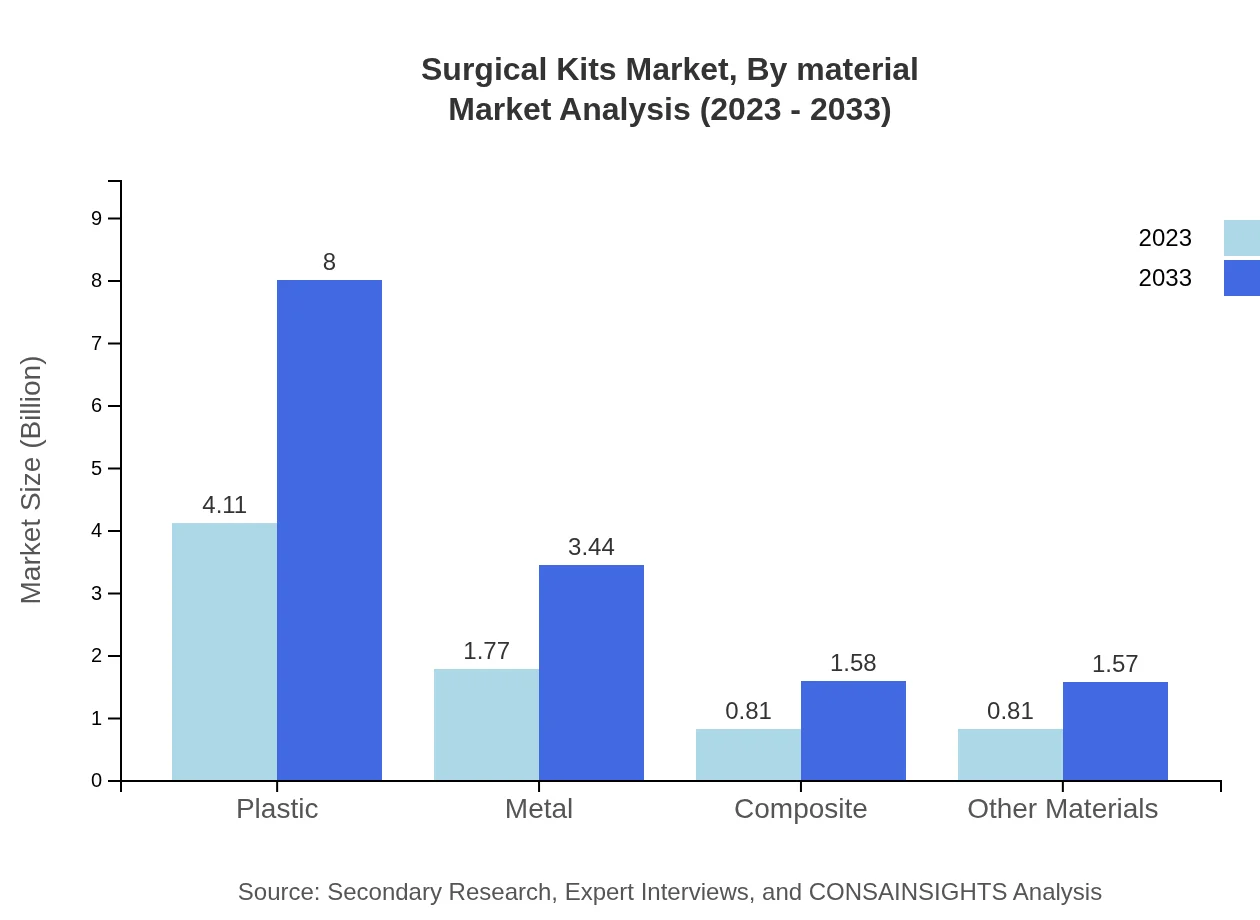
Surgical Kits Market Analysis By Material
The materials used in surgical kits encompass plastics, which hold the largest market size of $4.11 billion in 2023, metal ($1.77 billion), composite ($0.81 billion), and other materials ($0.81 billion). The plastic segment accounts for about 54.82% of the market due to its versatility in manufacturing disposable kits, while metal and composite materials are preferred for specific applications requiring enhanced durability and performance.
Surgical Kits Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Surgical Kits Industry
Medtronic :
Medtronic is a leading global medical technology company known for its innovative surgical products, including a comprehensive range of surgical kits designed for various medical applications.Johnson & Johnson:
Johnson & Johnson is a multinational corporation recognized for its diverse healthcare products including surgical kits that meet the highest safety and quality standards.3M Health Care:
3M Health Care creates innovative solutions for surgeries with its specially designed surgical kits that enhance surgical outcomes and patient safety.B. Braun:
B. Braun is renowned for its wide range of surgical kits and supplies, particularly in the field of anesthesia and infusion therapy, establishing strong partnerships with healthcare facilities.Cardinal Health:
Cardinal Health provides essential products and services to the healthcare sector, including a significant line of surgical kits tailored to meet the needs of diverse surgical specialties.We're grateful to work with incredible clients.









FAQs
What is the market size of surgical Kits?
The global surgical kits market is currently valued at approximately $7.5 billion, with a projected compound annual growth rate (CAGR) of 6.7% expected through the next decade.
What are the key market players or companies in this surgical Kits industry?
Major players in the surgical kits market include Medtronic, Johnson & Johnson, Cardinal Health, and Stryker, among others. These companies lead in innovation and supply comprehensive surgical solutions worldwide.
What are the primary factors driving the growth in the surgical kits industry?
Key drivers for growth in the surgical kits market include increasing surgical procedures globally, a rise in chronic diseases, and advancements in technology that improve surgical outcomes and reduce recovery times.
Which region is the fastest Growing in the surgical kits?
The fastest-growing region for the surgical kits market is Europe, with market expansion from $2.62 billion in 2023 to $5.09 billion by 2033, reflecting strong healthcare infrastructure and expenditure.
Does ConsaInsights provide customized market report data for the surgical Kits industry?
Yes, ConsaInsights offers customized market report data tailored to specific user needs, providing insights for targeted segments within the surgical kits industry.
What deliverables can I expect from this surgical Kits market research project?
Expect a comprehensive report detailing market size, growth forecasts, segmentation analysis, competitive landscape, and regional insights accompanied by actionable recommendations.
What are the market trends of surgical Kits?
Trends in the surgical kits market include a shift towards disposable kits for infection control, increased adoption of minimally invasive surgeries, and a focus on sustainability in materials and manufacturing.