Surgical Polypropylene Mesh Market Report
Published Date: 31 January 2026 | Report Code: surgical-polypropylene-mesh
Surgical Polypropylene Mesh Market Size, Share, Industry Trends and Forecast to 2033
This market report provides comprehensive insights into the Surgical Polypropylene Mesh sector from 2023 to 2033, analyzing market size, growth projections, and regional dynamics, alongside industry trends and forecasts.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
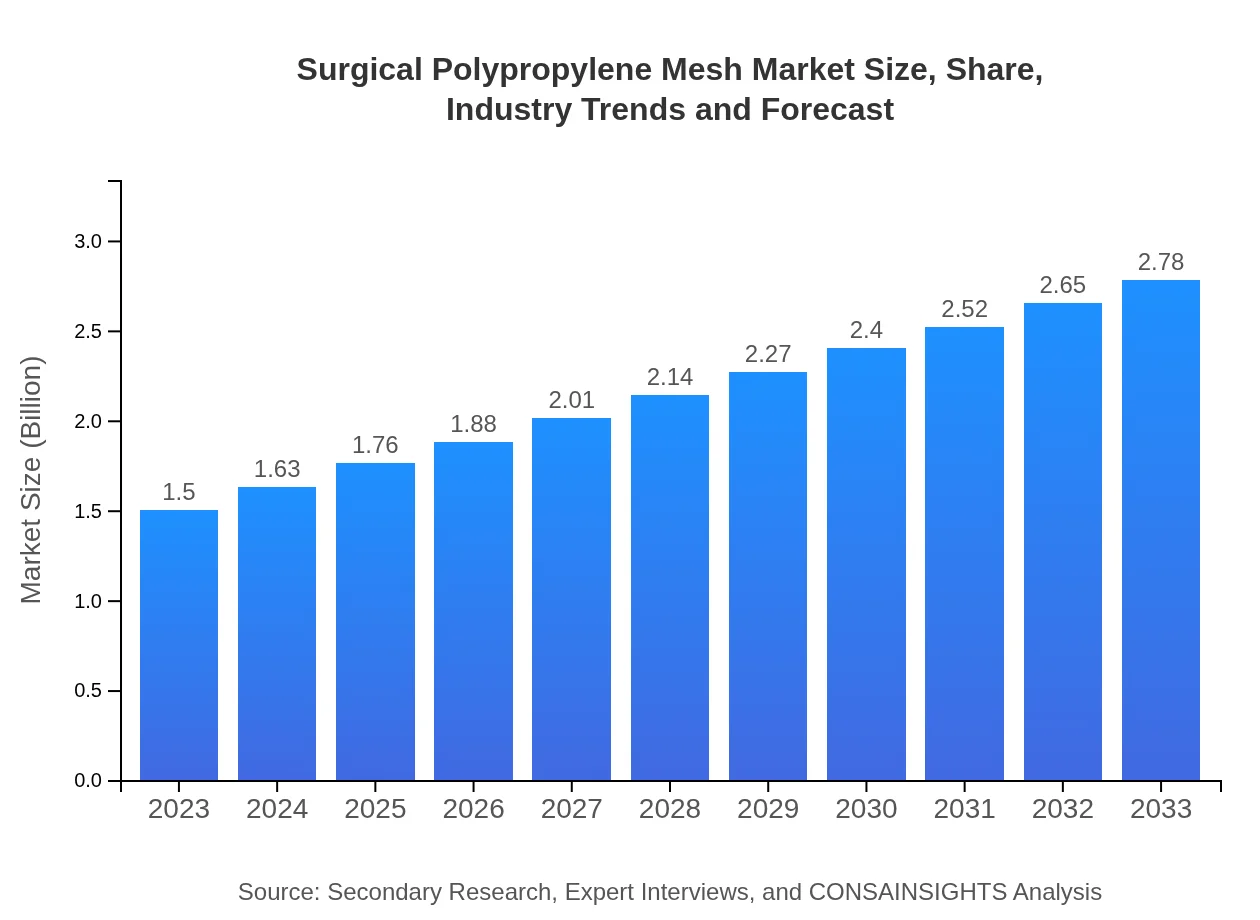
| 2023 Market Size | $1.50 Billion |
| CAGR (2023-2033) | 6.2% |
| 2033 Market Size | $2.78 Billion |
| Top Companies | Ethicon, Inc., Medtronic plc, Boston Scientific, Surgical Innovations Group, Bard Davol |
| Last Modified Date | 31 January 2026 |
Surgical Polypropylene Mesh Market Overview
Customize Surgical Polypropylene Mesh Market Report market research report
- ✔ Get in-depth analysis of Surgical Polypropylene Mesh market size, growth, and forecasts.
- ✔ Understand Surgical Polypropylene Mesh's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Surgical Polypropylene Mesh
What is the Market Size & CAGR of Surgical Polypropylene Mesh market in 2023?
Surgical Polypropylene Mesh Industry Analysis
Surgical Polypropylene Mesh Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Surgical Polypropylene Mesh Market Analysis Report by Region
Europe Surgical Polypropylene Mesh Market Report:
In Europe, the market is valued at $0.44 billion in 2023, with projections to reach $0.81 billion by 2033. The region is witnessing substantial investments in healthcare innovations, especially in surgical techniques and mesh technology.Asia Pacific Surgical Polypropylene Mesh Market Report:
In the Asia Pacific region, the Surgical Polypropylene Mesh market is valued at approximately $0.31 billion in 2023, projected to grow to $0.57 billion by 2033. The increased healthcare spending and technological advancements in surgical procedures are significant contributors to this growth.North America Surgical Polypropylene Mesh Market Report:
North America represents a substantial portion of the market, with a market value of $0.53 billion in 2023, projected to experience growth to $0.98 billion by 2033. Factors influencing this growth include high healthcare expenditure and a well-established surgical practice environment.South America Surgical Polypropylene Mesh Market Report:
The South American market is relatively smaller, with a value of $0.03 billion in 2023, expected to rise to $0.06 billion by 2033. This growth is attributed to an increase in surgical interventions and the expansion of healthcare infrastructure.Middle East & Africa Surgical Polypropylene Mesh Market Report:
The Middle East and African market stands at $0.20 billion in 2023, expected to grow to $0.36 billion by 2033. Growth drivers include improving healthcare access and increasing surgical procedures within the region.Tell us your focus area and get a customized research report.
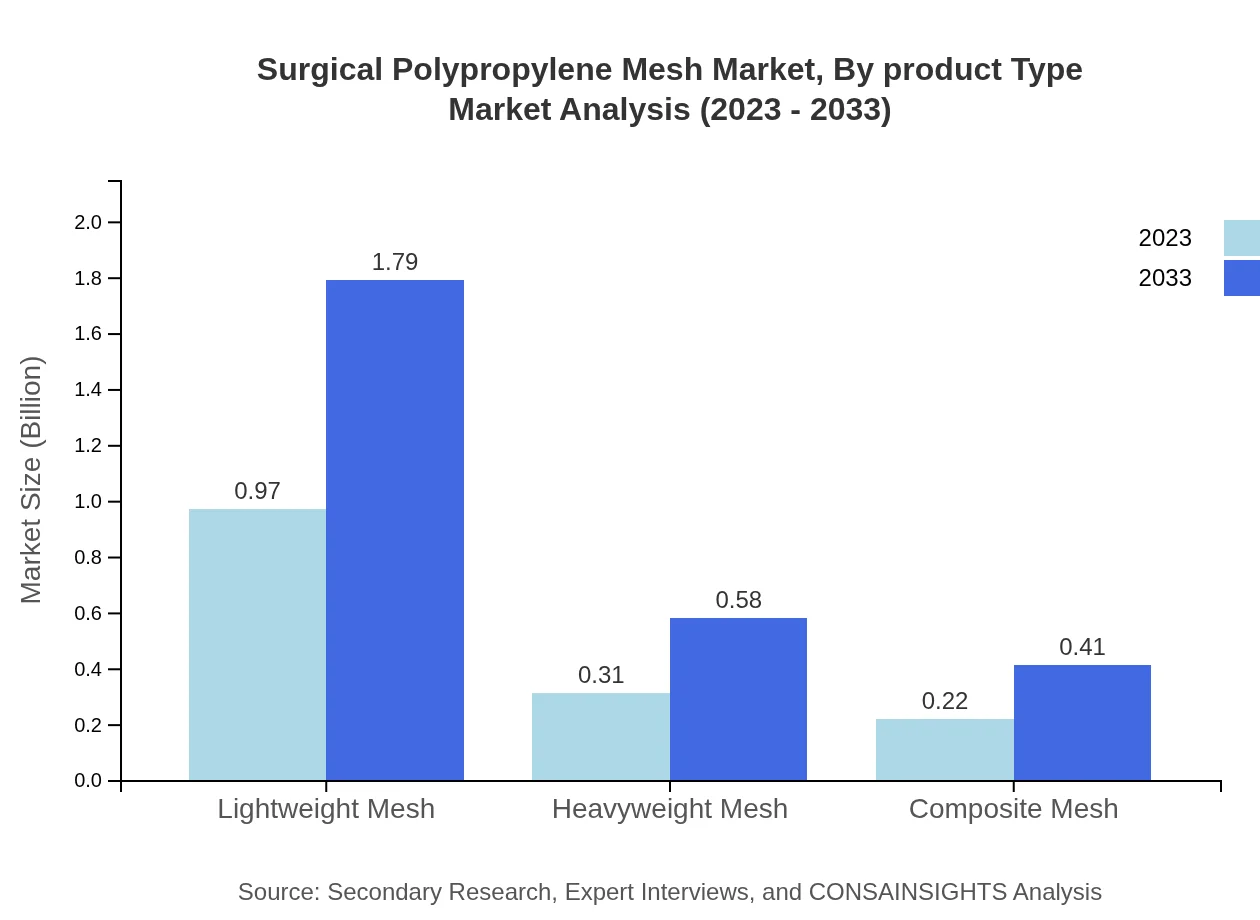
Surgical Polypropylene Mesh Market Analysis By Product Type
The product types in the Surgical Polypropylene Mesh market include Lightweight Mesh, Heavyweight Mesh, and Composite Mesh. Lightweight Mesh holds the largest market share of approximately 64.52% in 2023, indicating its preference for reducing recovery time and complications, followed by Heavyweight Mesh at 20.9%. Composite Mesh, though smaller, contributes to innovations, enhancing durability and performance.
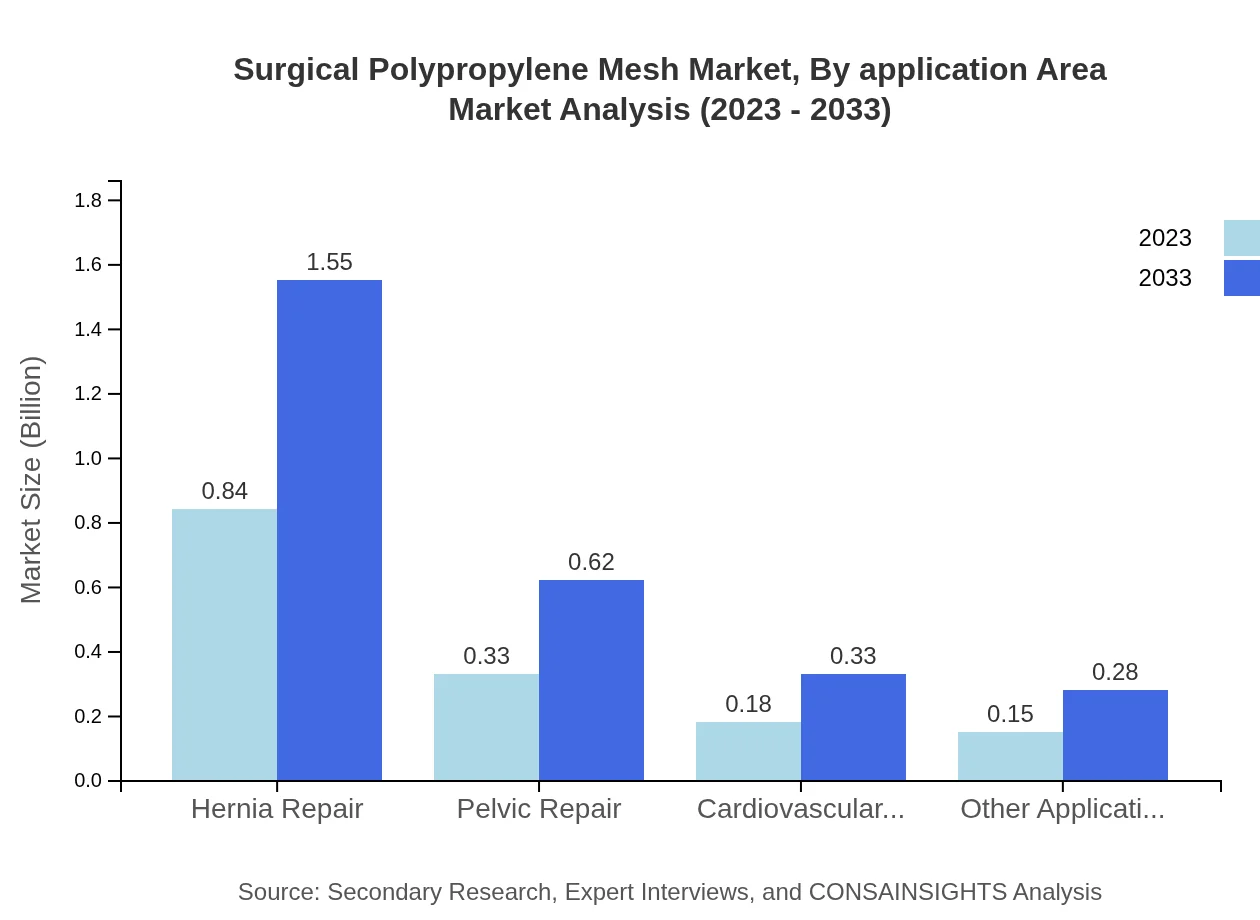
Surgical Polypropylene Mesh Market Analysis By Application Area
The major application areas for Surgical Polypropylene Mesh are Hernia Repair, Pelvic Repair, and Cardiovascular Surgeries. Hernia Repair is the leading segment, accounting for 55.76% of the market in 2023, supported by increasing hernia surgeries. The Pelvic Repair segment and Cardiovascular Surgeries are also growing steadily, reflecting the expanding surgical landscape.
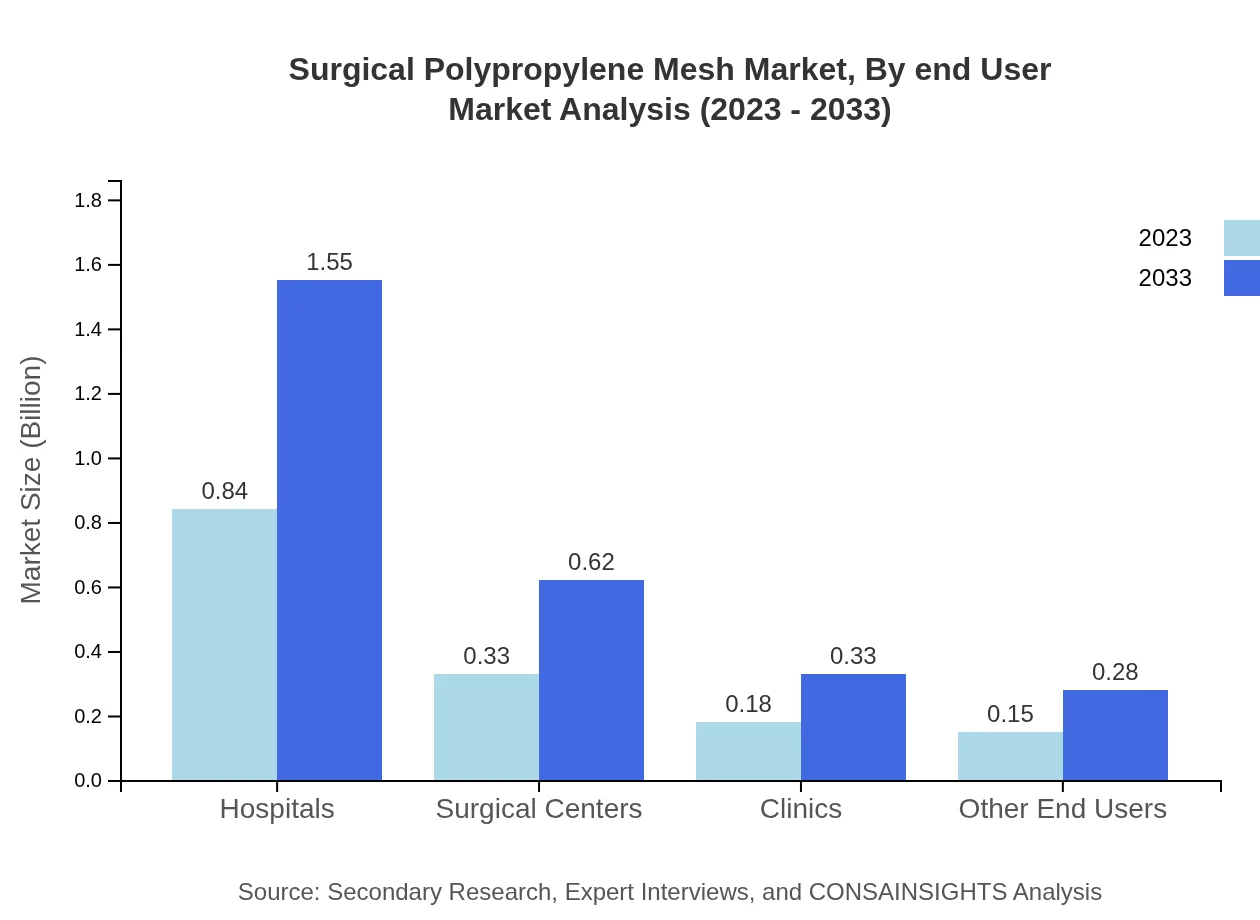
Surgical Polypropylene Mesh Market Analysis By End User
Hospitals remain the primary end-user for Surgical Polypropylene Mesh, with a share of 55.76% in 2023, indicating their reliance on cost-effective and efficient surgical materials. Surgical centers and clinics follow, contributing significantly to the demand as they increasingly adopt advanced surgical procedures focusing on patient outcomes.
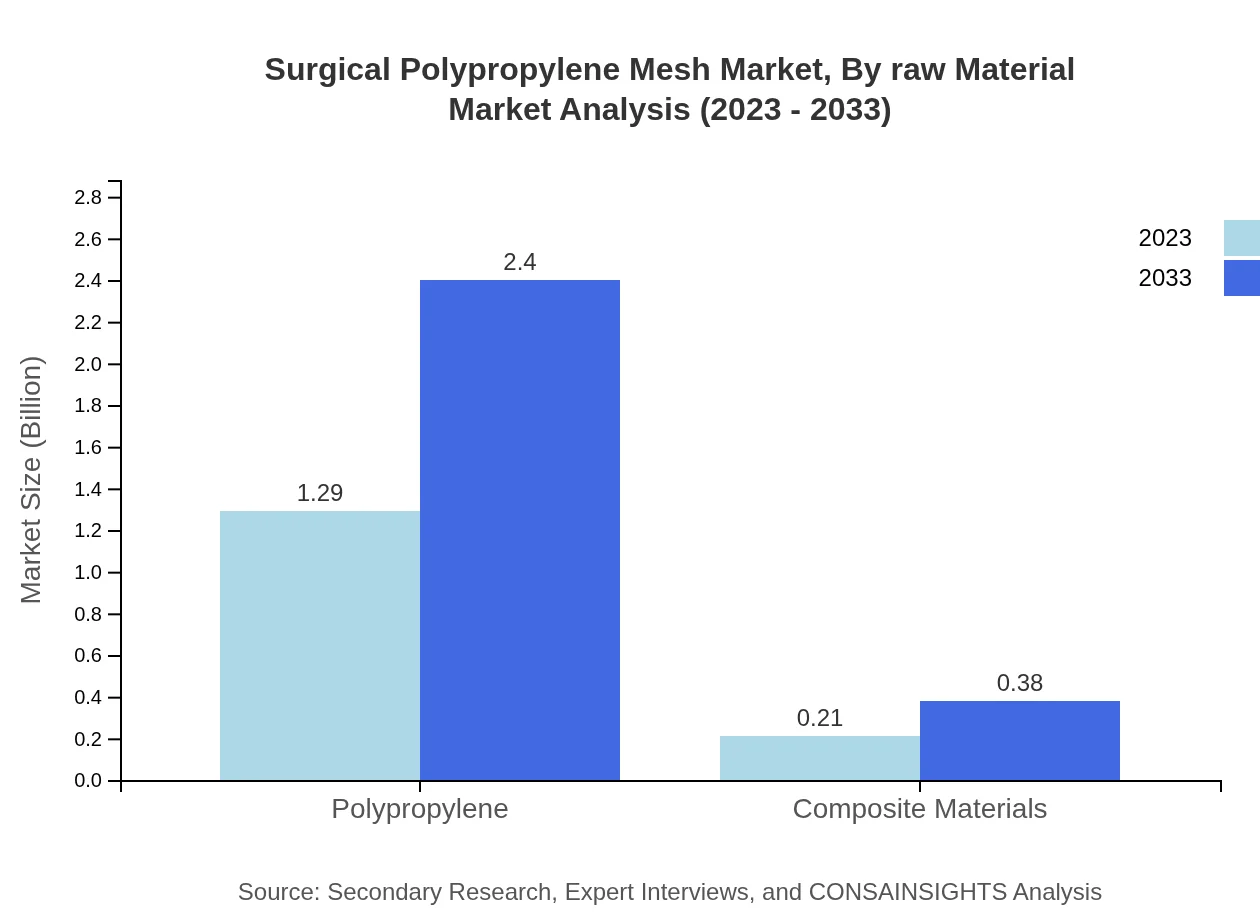
Surgical Polypropylene Mesh Market Analysis By Raw Material
Polypropylene dominates the raw material segment, accounting for 86.31% of the market in 2023, primarily due to its favorable properties and biocompatibility. Composite Materials also play a crucial role, representing 13.69% of the raw materials used, reflecting the growing shift towards innovative solutions in surgical applications.
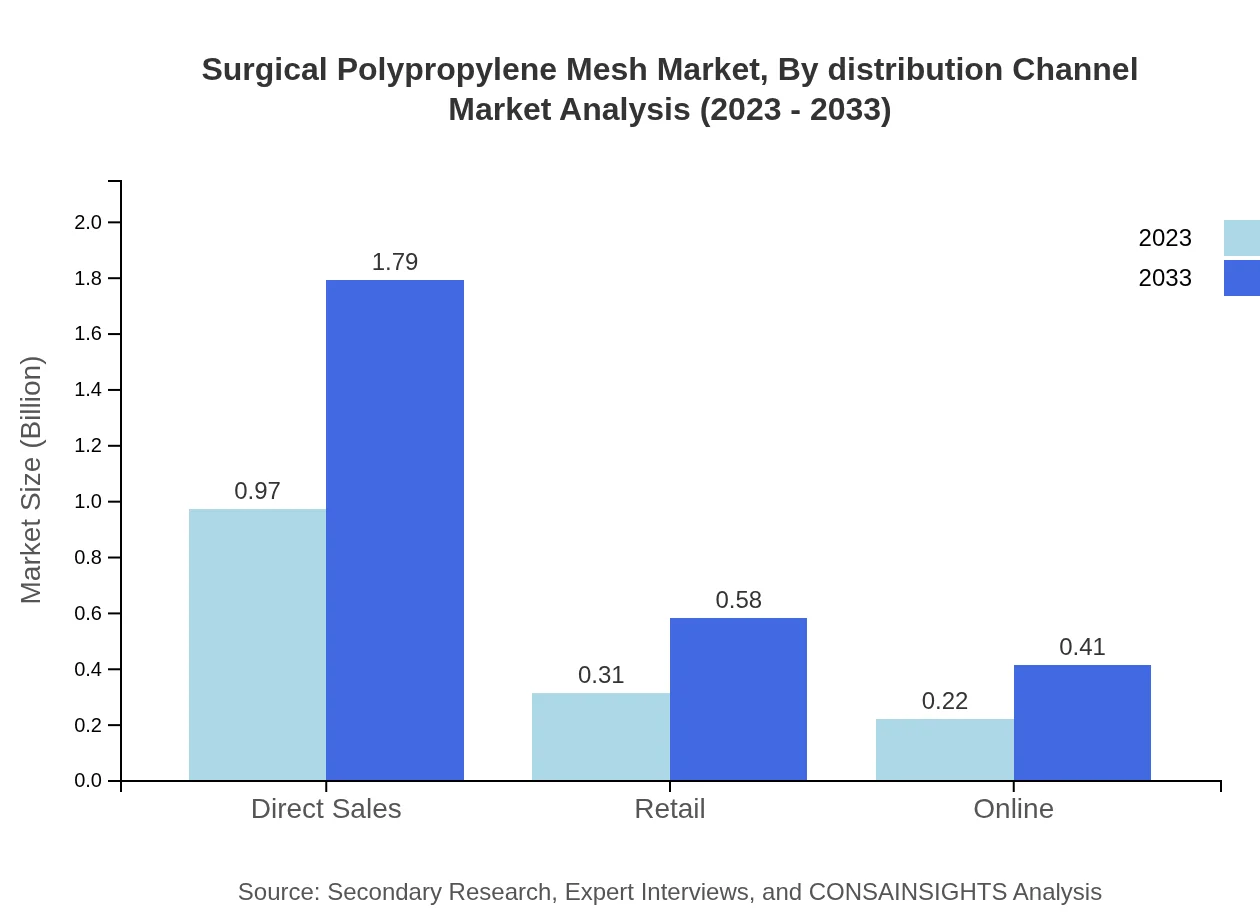
Surgical Polypropylene Mesh Market Analysis By Distribution Channel
Direct Sales channels dominate the market, representing 64.52% of the revenue in 2023, allowing manufacturers to build direct relationships with healthcare providers. Retail and Online distribution channels also show potential for growth, with increasing accessibility to products across the medical device marketplace.
Surgical Polypropylene Mesh Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Surgical Polypropylene Mesh Industry
Ethicon, Inc.:
As a leader in surgical solutions, Ethicon provides innovative polypropylene mesh products for hernia repair and tissue reconstruction, emphasizing safety and clinical efficacy.Medtronic plc:
Medtronic is a global leader in medical technology, focusing on developing advanced surgical meshes with a commitment to improving patient outcomes.Boston Scientific:
Boston Scientific offers a diverse range of surgical products, including polypropylene mesh, leveraging technology to enhance surgical performance.Surgical Innovations Group:
Known for their innovative approach, Surgical Innovations is a significant player in minimally invasive surgical products, including advanced mesh solutions.Bard Davol:
Bard Davol is widely recognized for its high-quality surgical products, including a range of polypropylene meshes for various medical applications.We're grateful to work with incredible clients.









FAQs
What is the market size of surgical Polypropylene Mesh?
The global surgical polypropylene mesh market is valued at approximately $1.5 billion in 2023, with an expected CAGR of 6.2%. Projections indicate substantial growth by 2033, bolstering its significance in the medical device sector.
What are the key market players or companies in this surgical Polypropylene Mesh industry?
Major players in the surgical polypropylene mesh market include companies like Ethicon, Medtronic, and Atrium Medical. These companies focus on innovation, quality, and regulatory compliance to capture significant market shares and drive competitiveness.
What are the primary factors driving the growth in the surgical Polypropylene Mesh industry?
Key growth drivers include the rising incidence of hernia repairs, advancements in surgical techniques, and increasing demand for minimally invasive surgeries. Furthermore, a growing aging population and awareness regarding surgical mesh benefits are propelling market expansion.
Which region is the fastest Growing in the surgical Polypropylene Mesh?
The Asia Pacific region is poised to be the fastest-growing market for surgical polypropylene mesh, with size expectations rising from $0.31 billion in 2023 to $0.57 billion by 2033, attributed to increasing healthcare infrastructure and surgical procedure volumes.
Does ConsaInsights provide customized market report data for the surgical Polypropylene Mesh industry?
Yes, ConsaInsights offers tailored market report data specific to the surgical polypropylene mesh industry. This includes detailed analyses, forecasts, and insights to meet unique client requirements, enhancing decision-making capabilities.
What deliverables can I expect from this surgical Polypropylene Mesh market research project?
Deliverables include comprehensive market analysis reports, growth forecasts, competitive landscape insights, trend analyses, and regional breakdowns of market segments, ensuring stakeholders have a clear understanding of the surgical polypropylene mesh market.
What are the market trends of surgical Polypropylene Mesh?
Current trends include increased utilization of lightweight mesh products, innovation in composite materials, and a shift towards online sales channels. Additionally, hospitals are emerging as primary end-users due to the growing demand for effective surgical solutions.